Fluorescence - Light Microscopy
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
12 Terms
Define Fluorescence?
(one question in the exam)
Fluorescence microscopy images cells or molecules that have been tagged with a fluorescent dye. The fluorescent substances are called fluorophores, which are integrated into the sample.
what are the limitations of Brightfield optical microscopy?
The main limitations of bright field microscopy include: low contrast for weakly absorbing samples, requiring staining of most specimens to be visible, potential damage to living cells due to intense light, difficulty visualizing transparent or similar-colored samples, and limited resolution due to the nature of light itself, making it challenging to see fine details in a specimen without proper staining
What is fluorescence?/ how does it work?
(will be in the exam!)
Fluorescence is a member of the ubiquitous luminescence family of processes in which susceptible molecules emit light from electronically excited states created by either a physical (for example, absorption of light), mechanical (friction), or chemical mechanism.
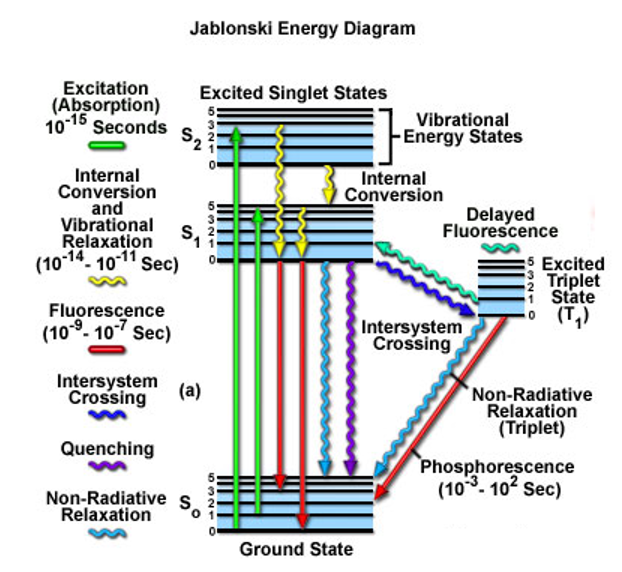
Luminescence processes are describe by the Jablonski diagram:
looking at molecules, molecular orbitals.
ground state are the electrons sitting on the outside, highest energy levels, P orbitals (highest occupied molecular orbital)(HOMO state).
S1 - lowest unoccupied molecular orbital. (LUMO state) no electrons
S2 - (LUMO + 1)
excitation = S0 to S1, needs light to occur. AB —hv1→ AB* (excited state), can either go back to AB, giving off hv2 or become A+B.
Infrared spectroscopy looks at the vibrational energy states.
most of the fluorescent compounds are aromatic structures (benzene; single (sigma bonds) & double bonds(p orbitals/pie bonds))
continue (what is fluorescence?)
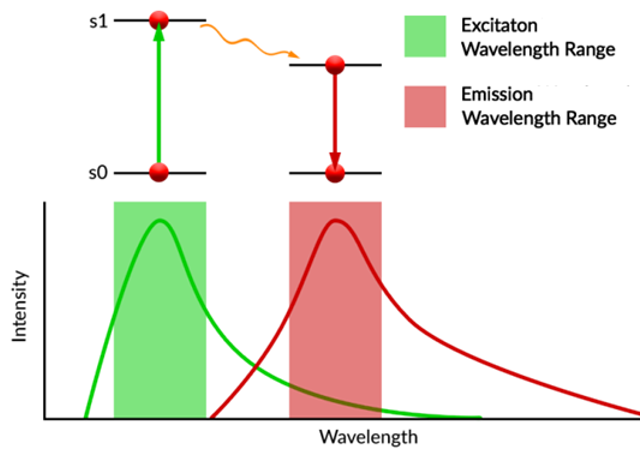
The spectral characteristics of a fluorescent molecule is they exhibit broad absorbance and emission spectra.
For highly fluorescence molecules the electronic excitation process is usually a π→π* transition found in highly conjugated planar molecules.
(*=excited state)
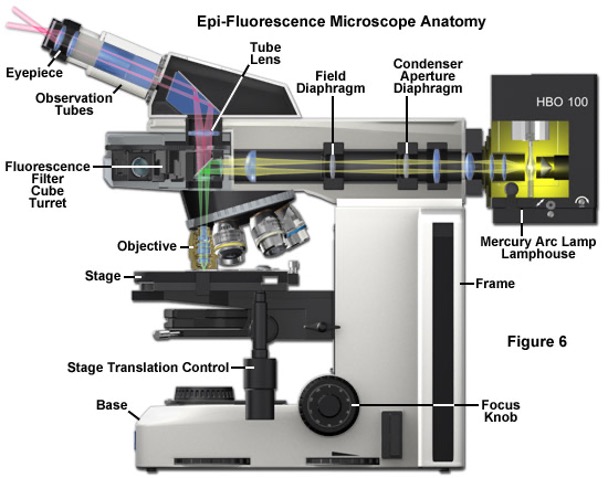
The microscope?
in a fluorescent microscope a mirror is used to shine the light in a specific place.
Epi-fluorescence microscopy is particularly useful when imaging thick samples, over 10µm deep.
However, the intense illumination and excitation of molecules outside the focal plane can produce images with a high background signal.

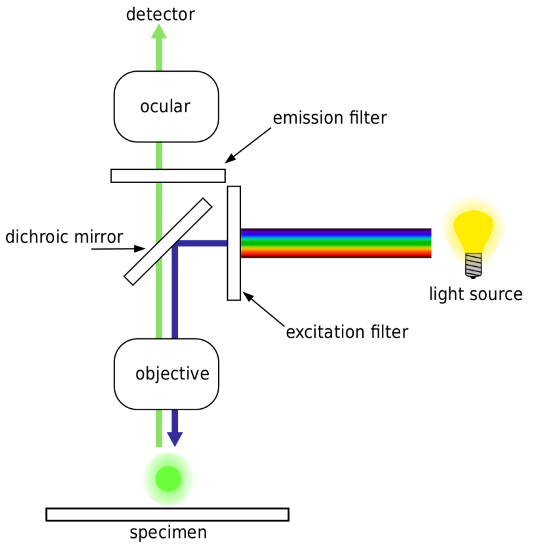
light source = white light
filter only allows blue light through
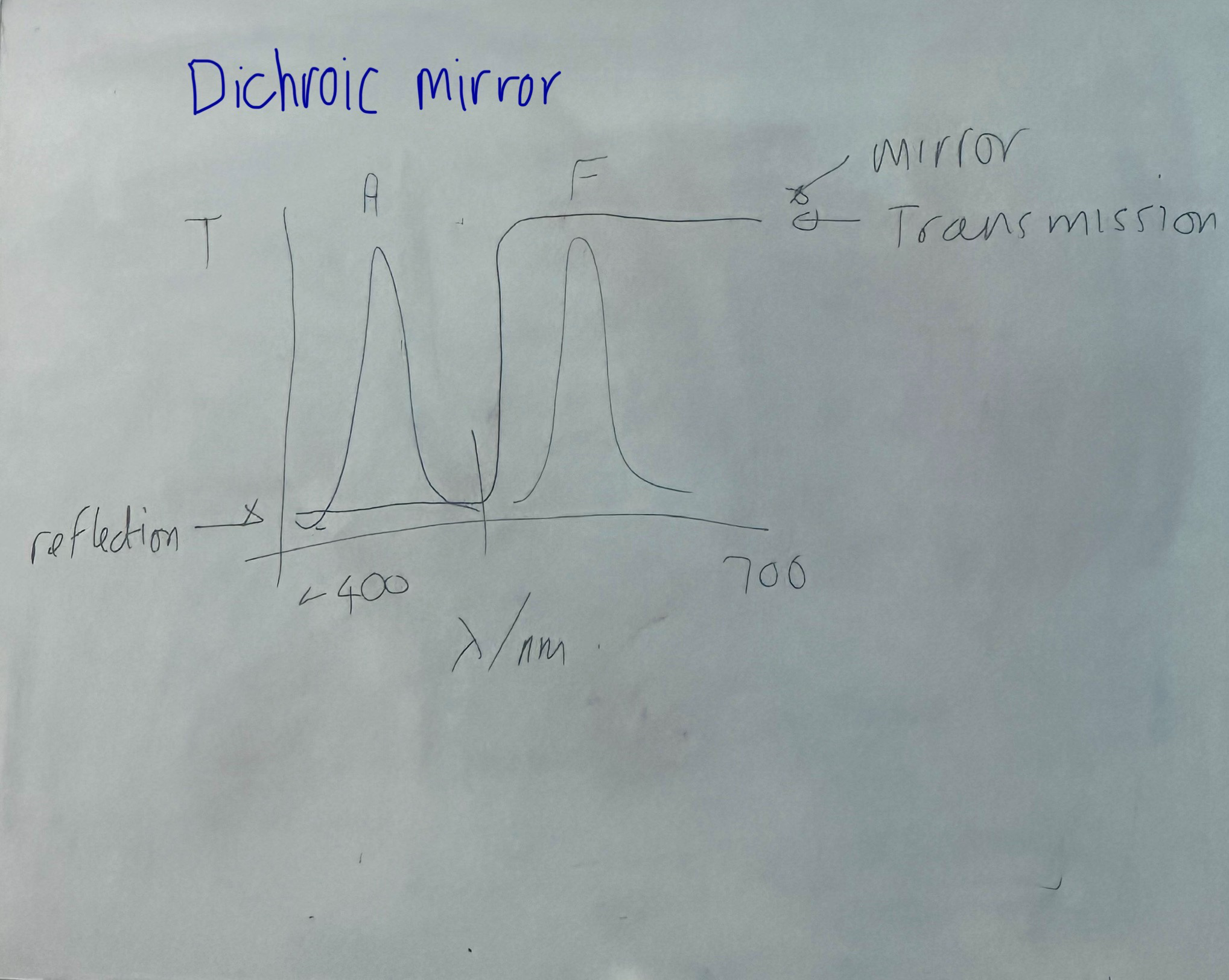
Dichroic mirror (wavelength cut off of around 475 between blue and green) transmits light through the objective and through the sample.
blue (around 400) Absorbance signal
green (around 550) F signal
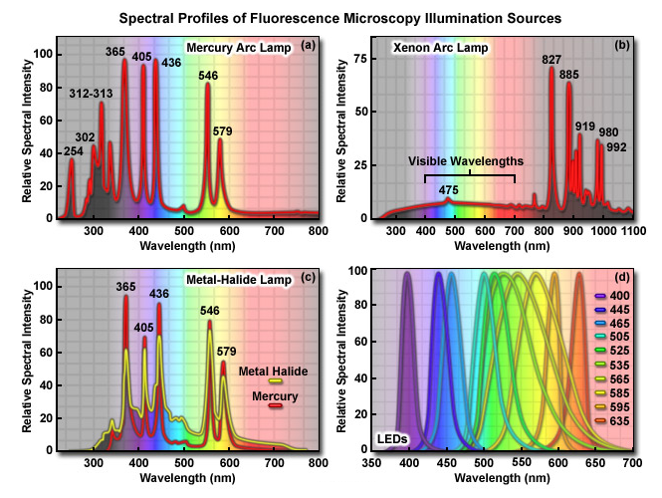
The primary consideration in choosing a light source for fluorescence microscopy is the spectral distribution in relation to the quantum yield and absorption of fluorophores being investigated.
In addition, the source must be compatible with the sensitivity of the detector used to capture images, whether it is the human eye, traditional film, a photomultiplier, intensified video tube, or a digital camera system.
In most reflected light illuminators, the excitation filter, dichromatic mirror, and barrier filter are incorporated into an optical block (often referred to as a cube).
Modern fluorescence microscopes are capable of accommodating between four and six fluorescence cubes.
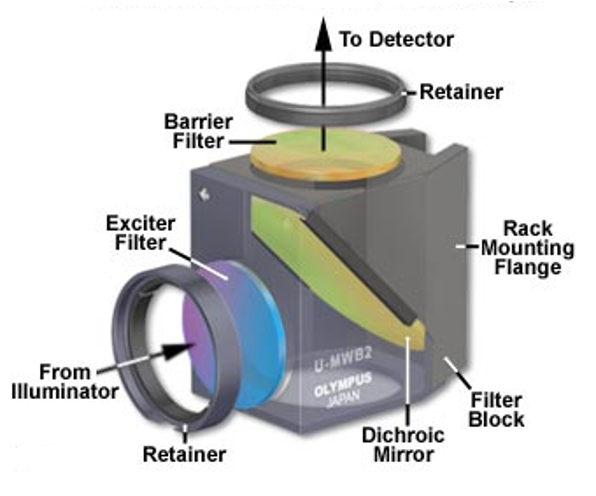
There are many objectives available from the manufacturers that are specifically designed for use with fluorescence illumination and thus feature high light transmission values in both the ultraviolet and visible portions of the spectrum.
The ‘fluor’ label indicate an objective suitable for fluorescence microscopy.

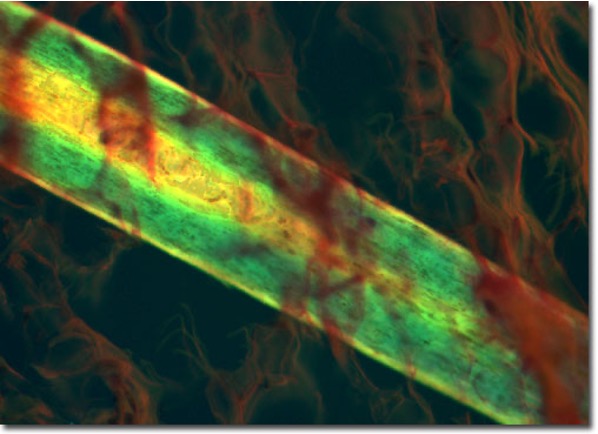
fluorescence microscopy images:
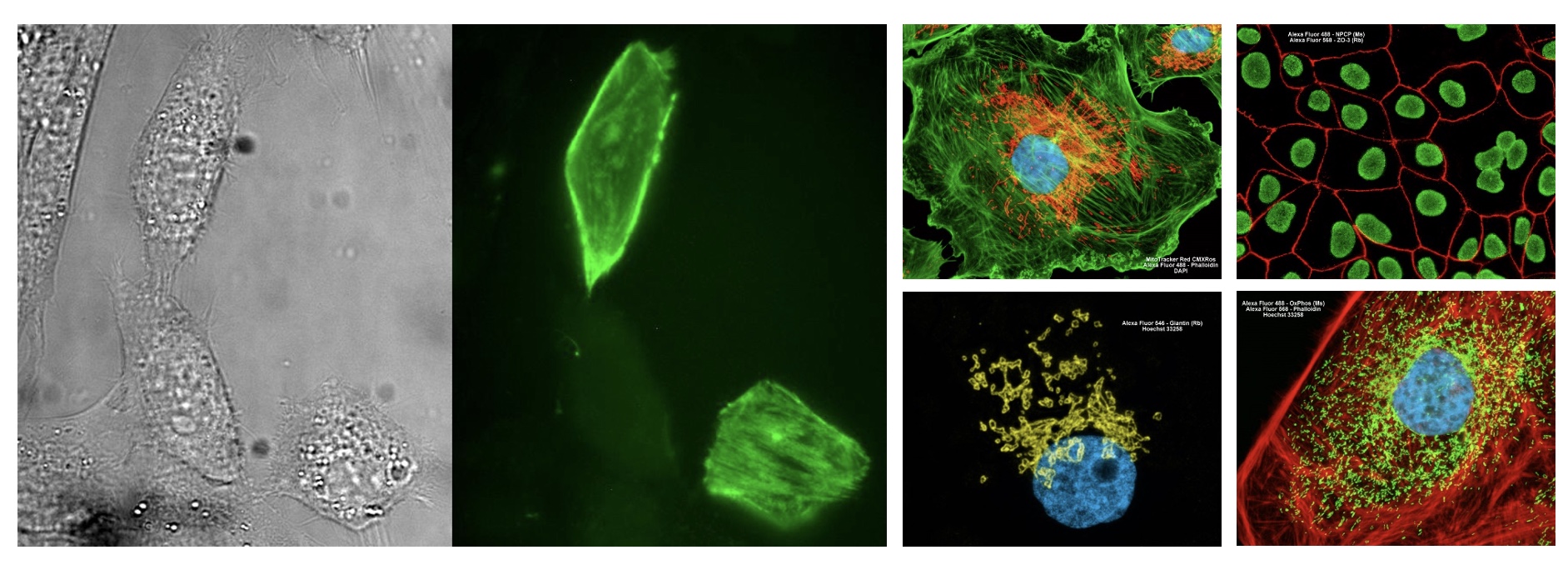
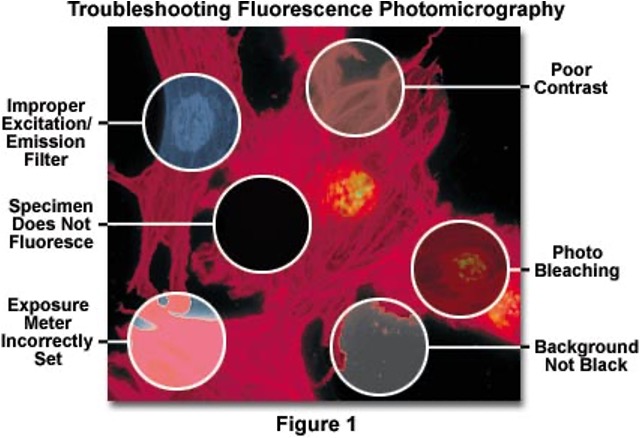
errors in fluorescence microscopy?