part 25: CHEMICAL KINETICS
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
31 Terms
Chemical Kinetics
Study of the rates of reactions and the mechanism by which these reactions occur
Degradation Rate
Rate of Reaction is also known as?
Rate of Reaction
The velocity with which the reactions occur
Depends on:
Reactant concentration
Temperature
pH
Presence of solvents or additives
Order of Reaction
The way in which the concentration of the drug or reactant in a chemical reaction affects the rate
Constant amount
Drug loss of Zero order
Constant fraction
Drug loss of First order
Independent
Concentration of Zero order
Dependent
Concentration of First order
conc/time
Unit of Zero order
per time
Unit of First order
Variable
Half-life of Zero order
Constant
Half-life of First order
Theophylline
Aspirin
Phenytoin
Ethanol
remember, “TAPE”
Example of drugs under Zero order
Most drugs!
Example of drugs under First order
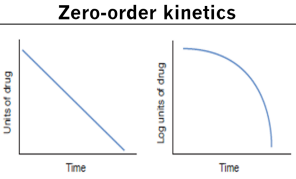
Zero order elimination
The same amount of drug is eliminated per unit of time
Regardless of the plasma conc of the drug, the rate of elimination is constant
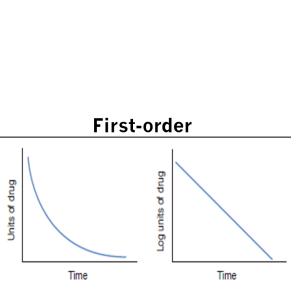
First order elimination
The same proportion of the drug is eliminated per unit of time
This leads to a variable amount eliminated based on the plasma conc
As the con drops, the elimination rate drops as well
Half-life
The period of time required for the concentration of a drug to decrease by one half
Shelf life
The period of time where 90 % of the original concentration is left
c = -kt +Co
General formula of Zero order reaction
Inc = -kt +InCo
General formula of First order reaction
t1/2 = 0.5 Co/K
Half-life of Zero order reaction
t1/2 = 0.693/K
Half-life of First order reaction
t90 = 0.1 Co/K
Shelf life of Zero order reaction
t90 = 0.105/K
Shelf life of First order reaction
conc/time
Units of k of Zero order reaction
per time
Units of k of First order reaction
89 mg/mL
Sample problem: CHEMICAL KINETICS
A suspension (125 mg/ml) decays by zero order kinetics with a reaction rate constant of 0.5 mg/ml/hr. What is the concentration of the active drug remaining after 3 days?
will remain after 120 days = 4.71 mg/mL
half-life = 1386 days
shelf life = 210 days
Sample problem: CHEMICAL KINETICS
An ophthalmic solution of a mydriatic drug at 5 mg/mL exhibits 1st order degradation with a k=0.0005/day. How much will remain after 120 days? Compute also for the half life and shelf life
3 mg/L
Sample problem: CHEMICAL KINETICS
If the plasma concentration just after a gentamicin dose is 10 mg/L and the patient's elimination rate constant is 0.15 hr-1, predict what the plasma concentration will be 8 hours later.
3 mg/L
Sample problem: CHEMICAL KINETICS
Using the equation C = C0e -Kt, determine the plasma concentration of a drug 24 hours after a peak level of 10 mg/L is observed if the elimination rate constant is 0.05 hr-1 .
7.5 mg/L
Sample problem: CHEMICAL KINETICS
For a drug that has an initial plasma concentration of 120 mg/L and a half-life of 3 hours, what would the plasma concentration be 12 hours after the initial concentration?