TOPIC 9 - POLYMERS UNFINISHED
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms
what is a polymer
a polymer is a substance of high average relative molecular mass made up of small repeating units
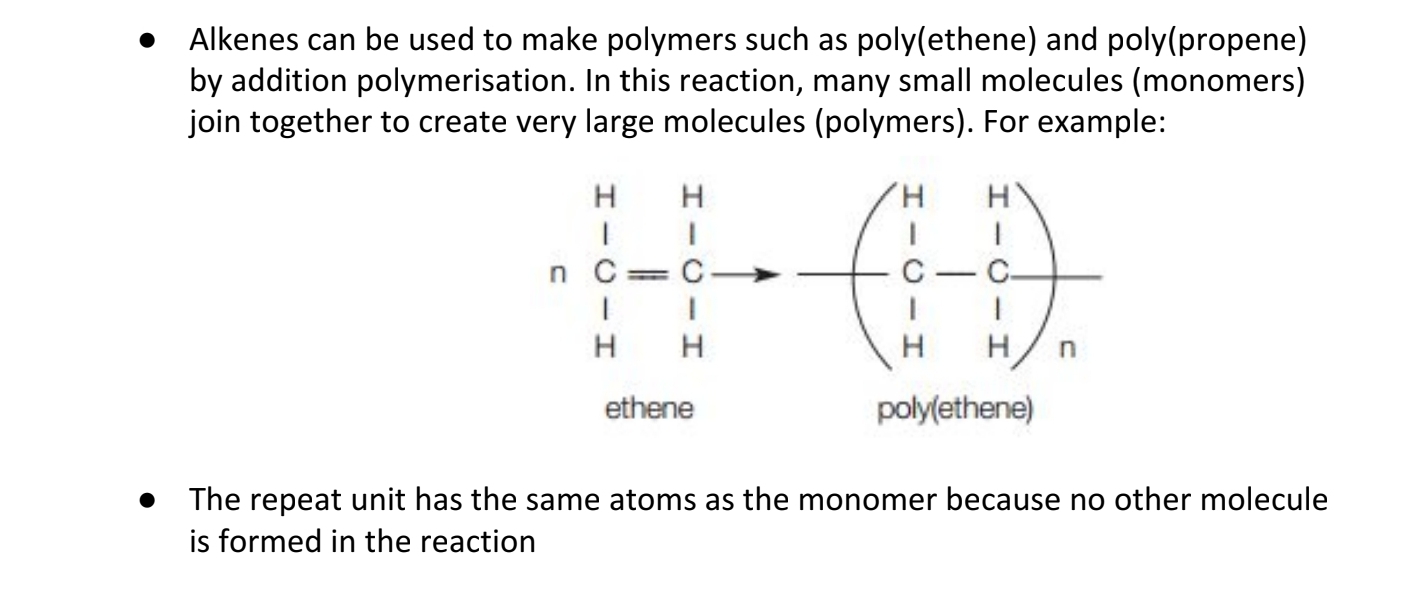
9.18C Describe: how ethene molecules can combine together in a
polymerisation reaction and that the addition polymer formed is called
poly(ethene) (conditions and mechanisms not required)
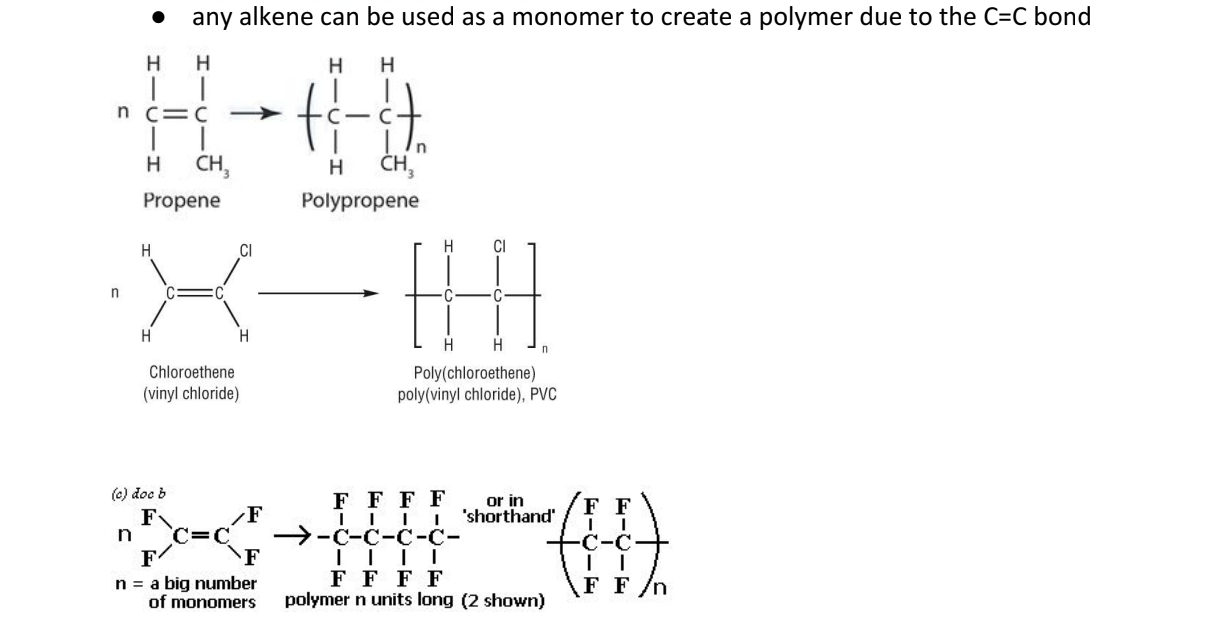
9.19C Describe how other addition polymers can be made by combining
together other monomer molecules containing C=C, to include
poly(propene), poly(chloroethene) (PVC) and poly(tetrafluoroethene) (PTFE)
(conditions and mechanisms not required)
educe the structure of a monomer from the structure of an
addition polymer and vice vers

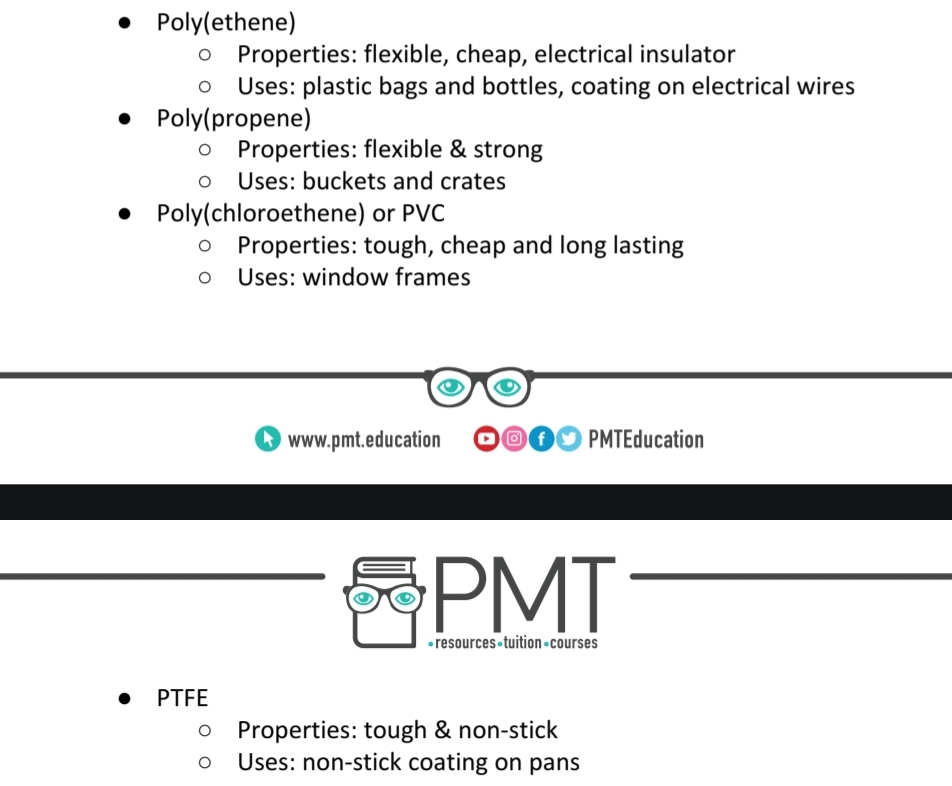
Explain how the uses of polymers are related to their properties
and vice versa: including poly(ethene), poly(propene),
poly(chloroethene) (PVC) and poly(tetrafluoroethene) (PTFE
Explain:
a why polyesters are condensation polymers
b how a polyester is formed when a monomer molecule
containing two carboxylic acid groups is reacted with a
monomer molecule containing two alcohol groups
c how a molecule of water is formed each time an ester
link is forme
9.23C Describe some problems associated with polymers
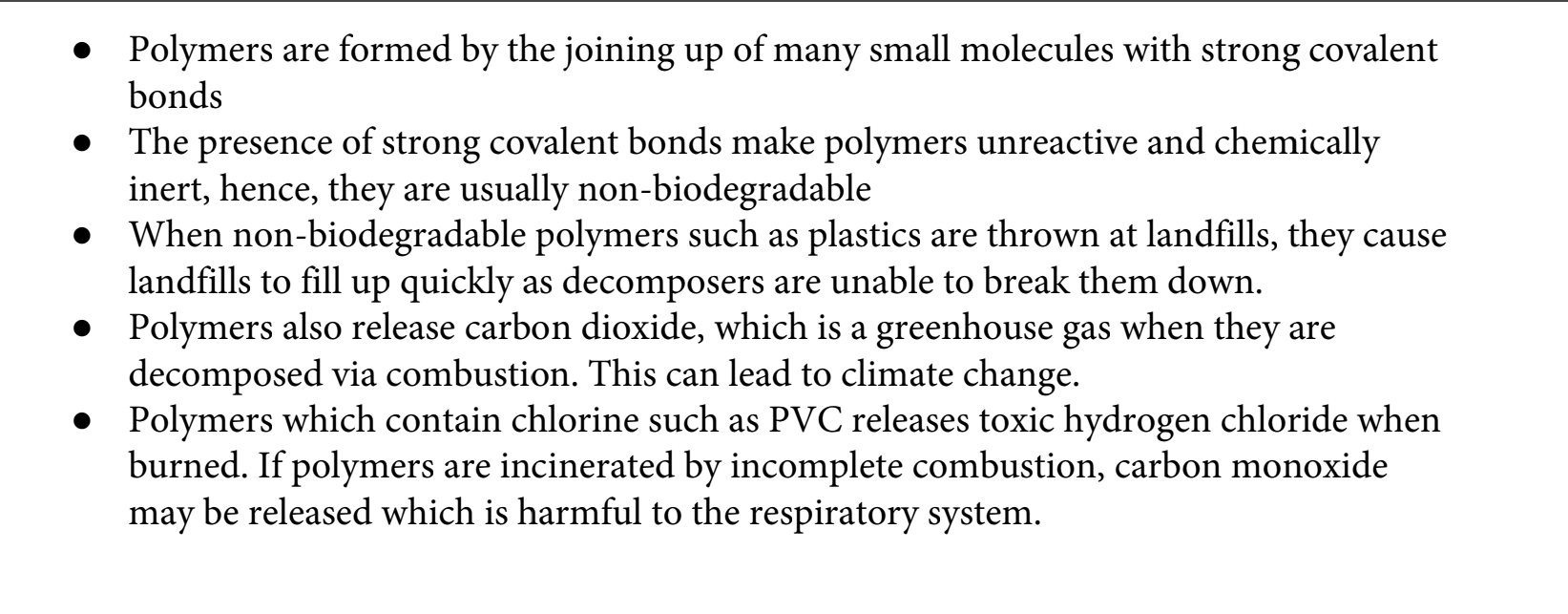
Evaluate the advantages and disadvantages of recycling
polymers, including economic implications, availability of
starting materials and environmental impact
