S1.4 Counting particles by mass: The mole
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
Avogadro’s constant
6.02 X 10²³
What is the relative atomic mass(Ar)
Weighted avg mass of one atom compared to 1/12th the mass of a carbon-12 atom
What is the difference between relative atomic mass and relative formula mass?
Atomic→one atom
Formula→compound or molecule
What is molar mass?
Same value as atomic mass
Measures mass present per 1 mole of specific element
How to convert particles to molar mass?
Avogadro’s particles→mole→molar mass
Formula triangle linking moles, particles and avogadro’s constant
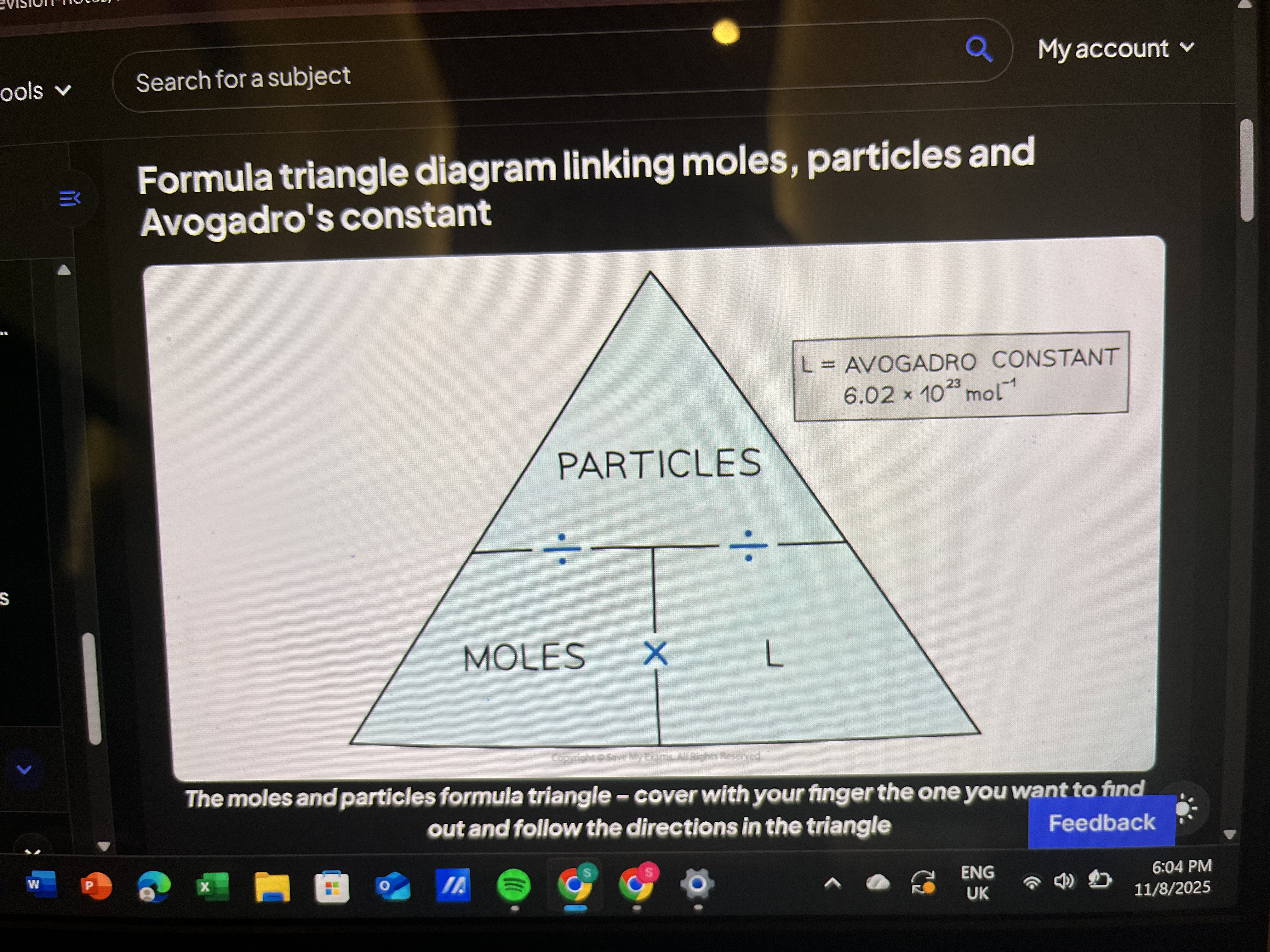
What’s the difference between molecular and empirical formula?
Molecular- formula of actual molecule
Empirical- simplest ratio of the formula
%composition formula
[(Mr of specific element x n)/Mr of molecule] x 100
Molar concentration(molarity formula)
c=n/V
(V always in dm³: 1L→1000cm³→1dm³)
How do u represent molar concentration
Use square brackets, eg:
[CuCl2]= 0.36M
Formula for mass concentration
c=m(g)/V
What r the stp conditions?
Temp→0°C(273K)
Pressure→1 atm(100kPa)
Relationship between n and V
2 gases will have same amt of particles if they occupy same volume at stp
n1/n2=V1/V2
(Can use mole ratio)