Buffer solutions
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
11 Terms
what is a buffer solution
resists change in pH when small amounts of acid or base added
2 requirments to be a buffer solutions
1] must contain undissolved weak acid (HA) and that acid’s conjugate base (A-)
way of making Buffer solution I
mix weak acid with salt of conjugate base in (s) or (aq)
way of making buffer solution II
partiually nuterilise a weak acid with a strong base
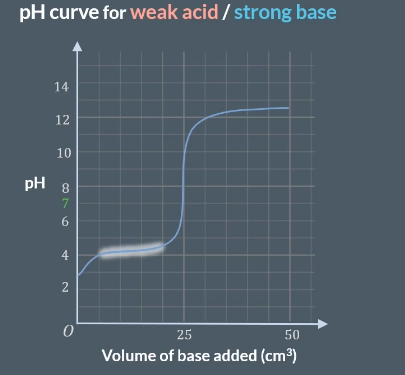
why so flat
so flat as acid partiually nuetrilsies so results in solution acting as a buffer solution where it resists changes in pH
how does a buffer solution resist change when acid is added
add acid , dissociates H+ , increase [H+] , equilibrium shifts left , decrease [H+] , [H+] stays roghly the same so pH stays oughly the same
how does a buffer solution resist changes when alkeline is added
add alkeiine , OH- reacts with H+ , decrease[H+] ,EQUILIBRIUM SHIFTS TO THE RIGHTS , INCREASE [H+] , [H+] roughly stys the same so pH rougly stays the same
everyday used of buffer solutions
swiiming pools , blood pH ,
acid buffer solution , PH and how to make
pH and how to makr acidic buffer solutions
weak acid + conjugate base
pH<7
pH and how to makr alkeline buffer solutions
weak base and conjugate base
pH>7