Preparation and properties of Ethyne
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
15 Terms
Draw a labelled diagram to show a suitable arrangement of apparatus and chemicals for the preparation of ethyne from the reaction of calcium carbide with water and for the collection of the gas in test-tubes over water
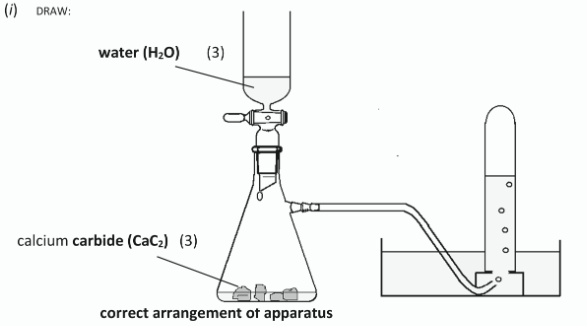
What was observed in the reaction flask as the water was dropped onto the calcium carbide (CaC2)?
Reaction mixture turns milky white / bubbling / vapour / effervescence / solid reacts / flask gets hot
Why would you expect the first test-tube of gas collected to be less pure than the others?
Air (oxygen, nitrogen) present
Explain why the first test-tubes of gas collected were discarded
Contain air
What was observed when a few drops of dilute bromine solution were added to one of the test-tubes of ethyne?
Brown decolourises (to colourless)
What was observed when a few drops of acidified, dilute potassium manganate(VII) solution were added to another one of the test-tubes of ethyne?
Purple decolourises (to colourless)
What information do the results of the two above tests give about ethyne?
Unsaturated / multiple bond
Describe how you would use one of the test-tubes of ethyne to confirm ethyne gas is flammable
Insert lighting paper into test-tube
Describe the flame observed when ethyne is burned in air
Bright (luminous) / yellow / sooty
Write a balanced equation for the combustion of ethyne in excess oxygen
2C2H5 + 5H2O → 4CO2 + 2H2O
How does the flame used in oxyacetylene welding, where ethyne is burned in pure oxygen, differ from the flame observed when ethyne was burned in air in a combustion test?
Hotter / cleaner (less sooty, less smoky) / less bright / blue
Compare the observations made in the two combustions tests of ethene and ethyne
Ethyne smokier (sootier, brighter, more luminous) than ethene’s /
ethene flame cleaner (not as bright, less luminous) than ethyne’s /
ethyne smoky (sooty) / ethene clean
Name a reagent used to test the gas for unsaturation
Bromine (Br2)
Write an equation for the reaction taking place during a test to show that ethyne is unsaturated. Name the organic product
C2H2 + Br2 → C2H2Br2
Dibromoethene
Give a major use of ethyne
Oxyacetylene flame / cutting metals / welding metals