Signal Transduction Ch. 11 videos 5&6
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
28 Terms
Calcium as secondary messenger
many pathways use calcium instead of cAMP or in addition to cAMP, as a secondary messenger
Ca+ is small, water-soluble, and freely diffusible, making it a good second messenger
There is a low concentration of it in the cytosol because Ca+ gets pumped out of the cell, as well as pumped inside storage organelles like the mitochondria or ER
When Ca+ concentration DOES rise in cytosol, any protein dependent on calcium concentration can become activated
Protein Kinase C is a protein responsive to calcium concentration
Where is Ca2+ located in the cell?
mitochondria or ER until the channel opens
phospholipase C
an enzyme linked to a pathway involving Ca ions.
can cut phospholipids
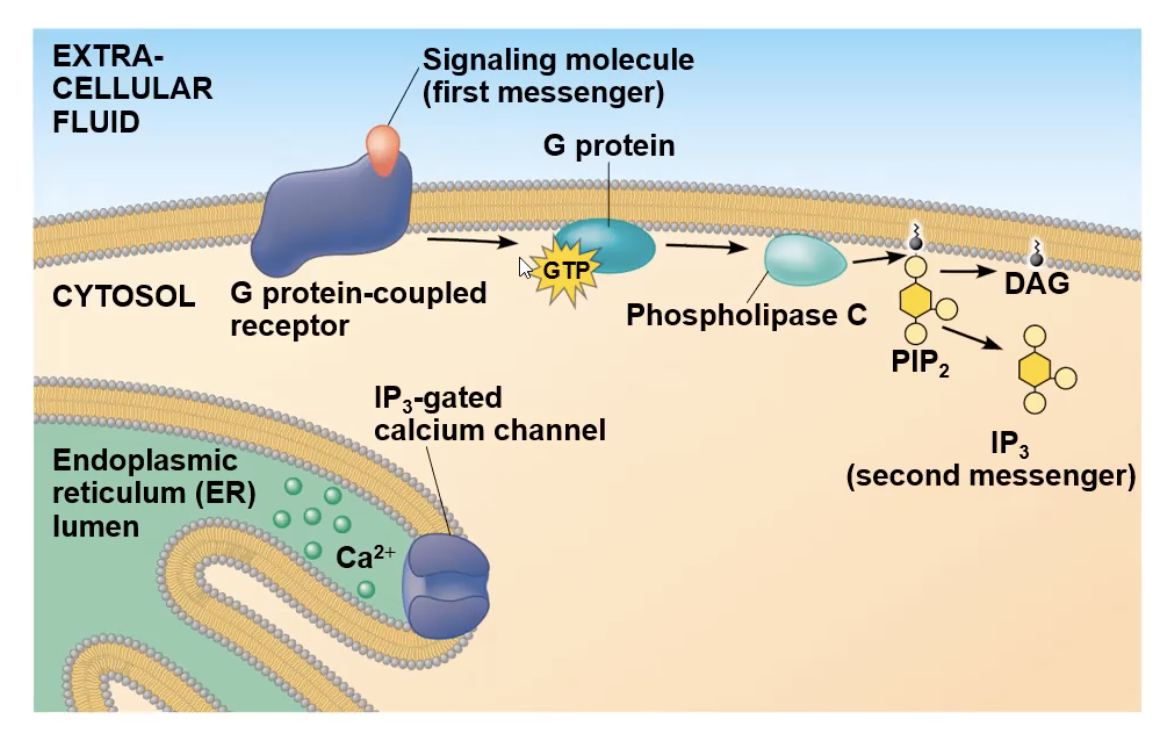
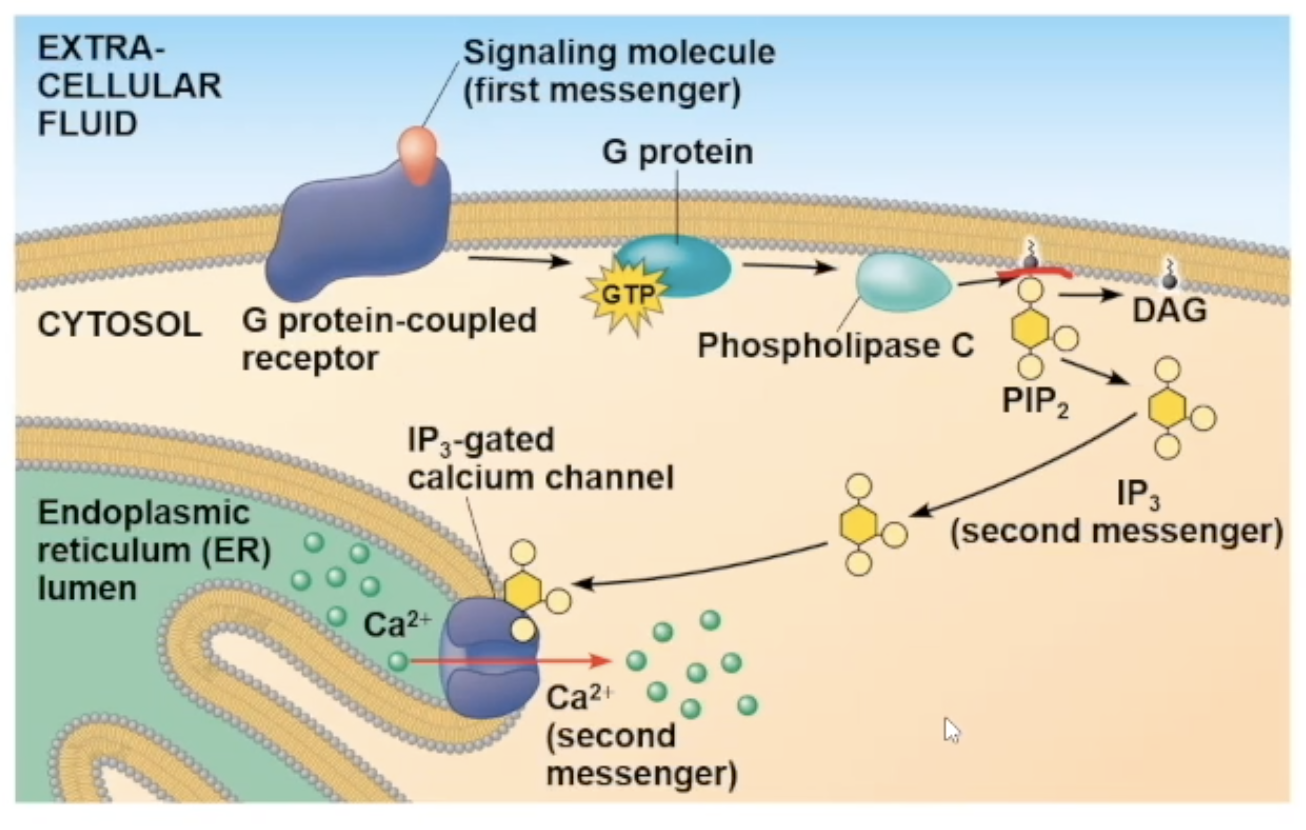
After first messenger binds to G protein receptor, the G protein will interact with the receptor and drop its GDP and gain GTP, which activates it to be mobile, and attach to phospholipase C.
Full Ca pathway
signal molecule attaches to receptor
receptor goes through a conformational change and will interact with G protein
interaction with G protein causes it to lose its GDP nad gain GTP, causing it to be mobile and travel to phospholipase C
phospholipase C activates, cuts PIP2 molecule creating IP3 molecule (the second messenger)
IP3 diffuses to a gated calcium channel and binds to it. the binding opens the channel
The channel opening allows the Ca2+ ions inside the ER to travel out to the cytosol
The ions can bind to many different protein types, and depending on what it bind to cause a specific cellular response
protein Kinase receptor
class of cell surface receptors that play a crucial role in signal transduction pathways
resposible for protein phosphorylation
a specific one we will focus on is the receptor tyrosine kinases (RTK)
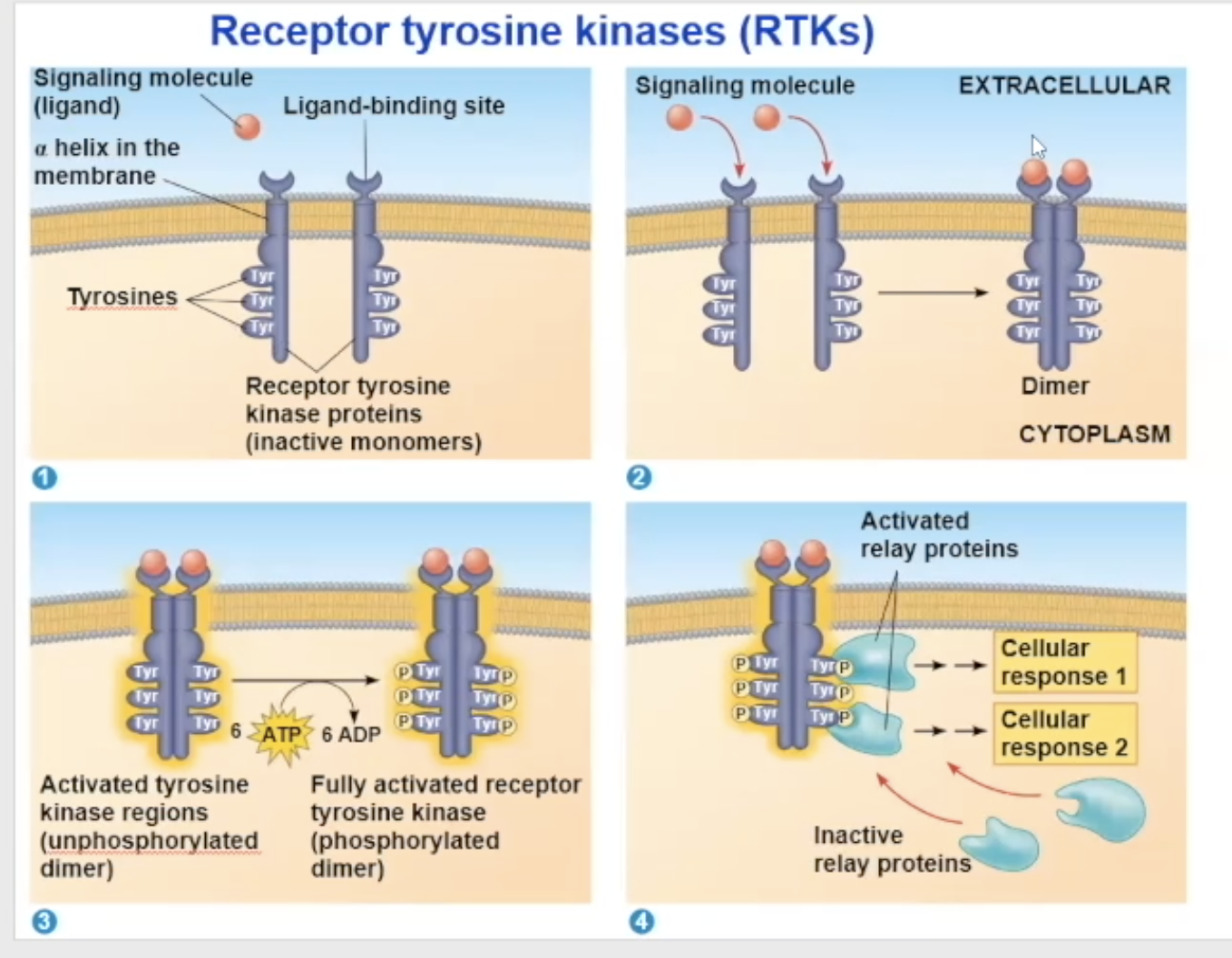
Receptor tyrosine kinase (RTK) structure
one RTK monomer has
a binding site that the ligand can bind to
a side in the cytoplasm that has RTK proteins on them to help with its enzymatic fuction
The side in the cytoplasm has tyrosine proteins on them
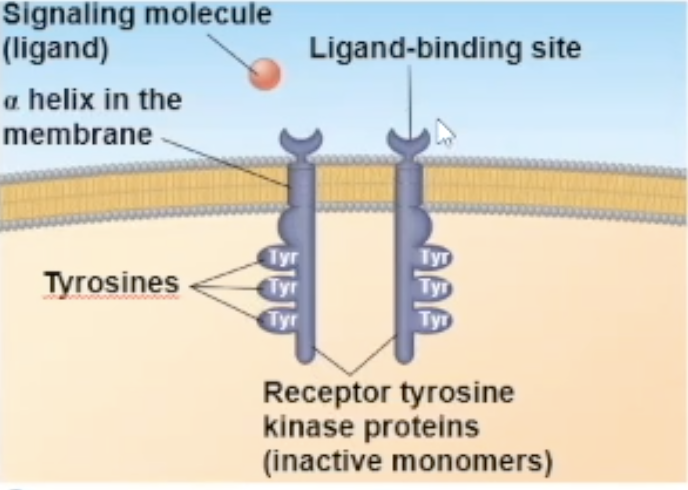
RTK binding
Either 2 signal molecules can bind to 2 independent RTK molecules, causing them to combine and turn into a dimer, OR 1 signal molecule can bring the two RTK molecules together and bind to both at the same time
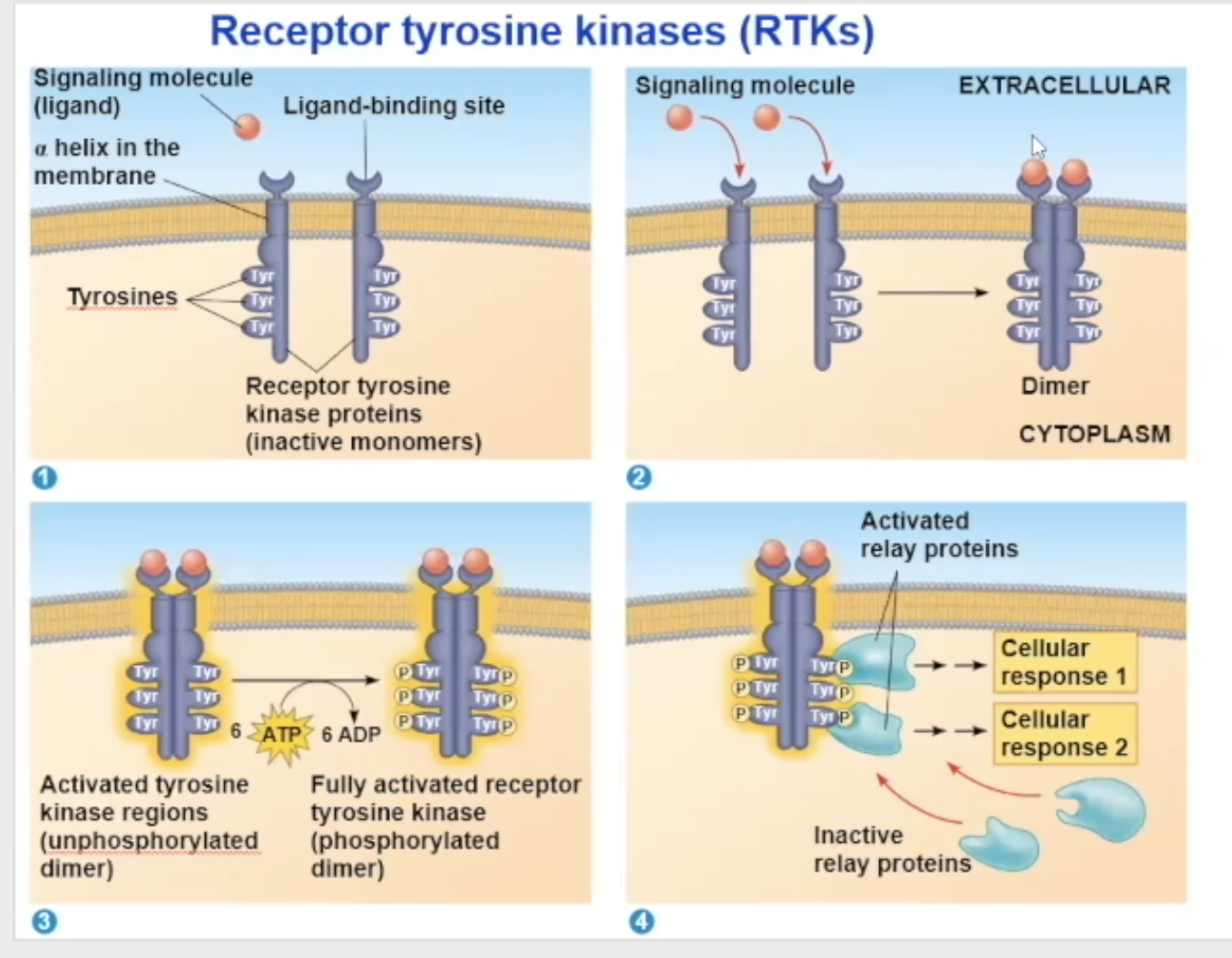
receptor tyrosine kinases (RTK) process
Their unactivated state is independent, but when activated, it combines with another RTK receptor
The dimerization activates the molecule and goes through autophosphorylation, which means the two monomers put phosphate groups on each other’s tyrosine proteins
the phosphates come from ATP, so at the end of the auto-phosphorylation, it converts its used ATP to ADP
Now in its fully phosphorylated form, the dimer can bind with relay proteins
the binding of the relay prtiens is the beginning of the tranduction
Scafollding peoteins
multiple protein kinases can combine together with a scaffolding protein
this increases the signal transduction efficiency because multiple proteins are already groped together and can bind to a receptor together
Termination of Signals
After a signaling molecule sends a signal which brings about a response, and then it needs a way to end the signal.
→ They can use
reversible binding
turning off enzymes
reversible binding
The two molecules (ligand and receptor) will join together and come apart
(The signaling molecule diffuses away from the receptor)
After inactivating all the proteins affected by the initial binding inactivates as well
for example an activated G protein with a GTP will turn off by hydrolyzing the GTP and gaining GDP
GTPase
when a G protein can hydrolyze its bound GTP (turning off on its own)
whena g protein is not a GTPase, it will get assistance from an enzyme to shut off
Termanation of signal- what happens to the ezyme
the protein will diffuse away from the enzyme, making it inactive again
all the molecules made by the enzyme gets destroyed
phosphodiesterase
An enzyme that destroys all the second messengers created by the enzyme after the enzyme is inactivated
What if the Termination of a signal does not occur? (example)
Vibrio cholerae (cholera) produces a toxin that modifies a G protein so its stuck in active form
this causes the protein to continually produce cAMP causing intestinal cells to secrete large amounts of salt into the intenstines
water follows by osmoses
an untreated person will lose salt and water and can die
how are cells different from eachother?
Cells are different from each other because they have different collections of proteins
They have different proteins within them because they have different genes being expressed
specificity of cell signaling
the arrival of the same signaling molecule in different cell types can bring about different effect
the different effects can be becuase
the proteins within the cells are different
a cross talk between two signal molecules binding can cause a new pathway
the signal molecule binds to a different receptor
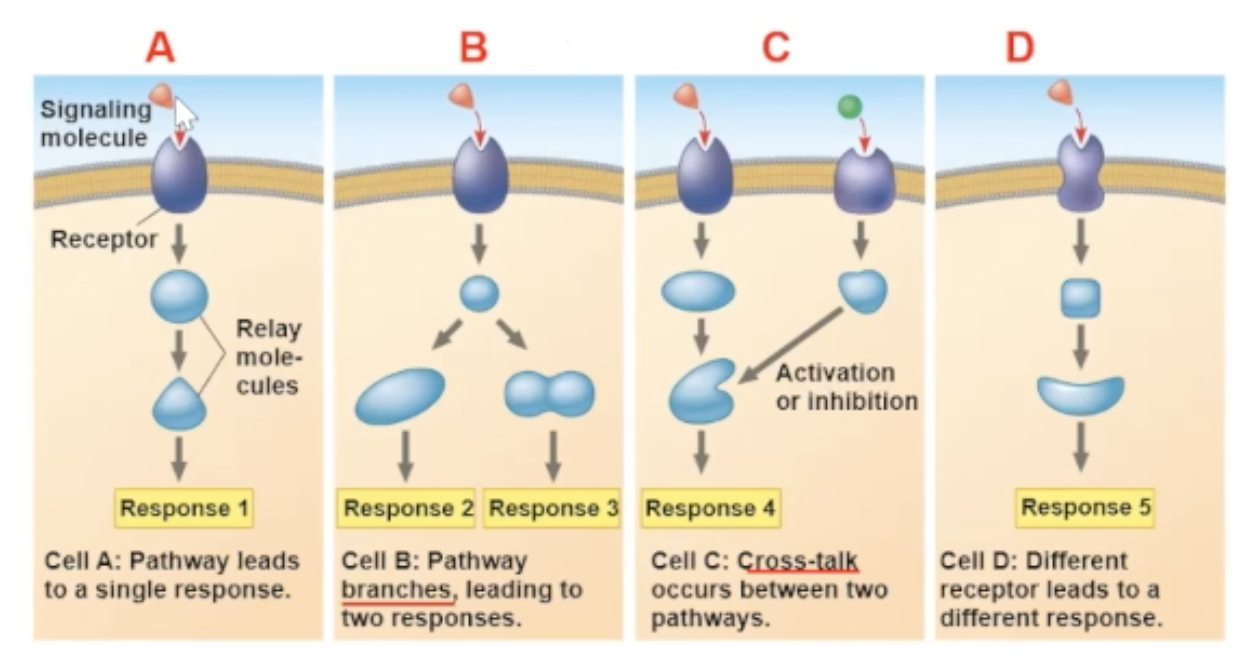
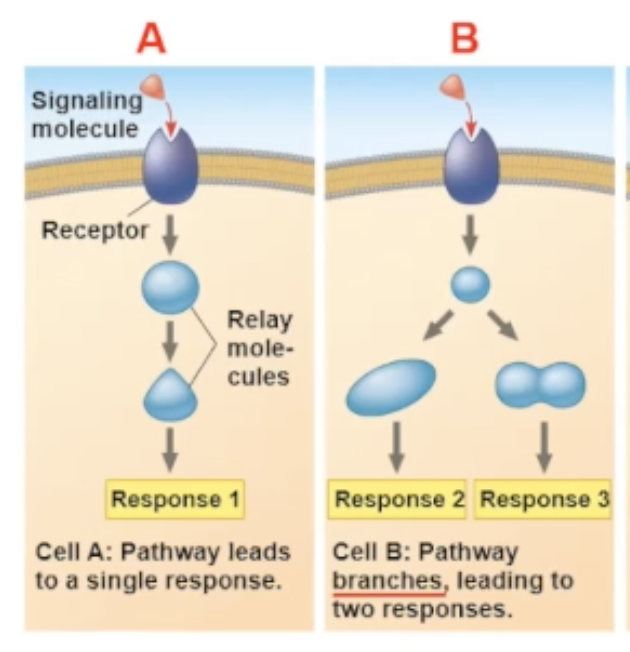
Why does the Same signal-molecule/receptor binding bring different pathways for different cell types?
The proteins in the cells are different.
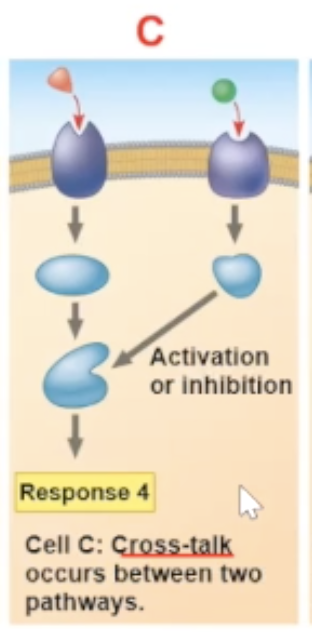
Cross-talk
a cross-talk is when two different signaling molecules bind to their receptors, and the result of the two binds occuring leads to a new path
a cross-talk is another reason why different cells can have different responces to the same signal molecule binding
different receptor
different receptors will communicate with different relay protein
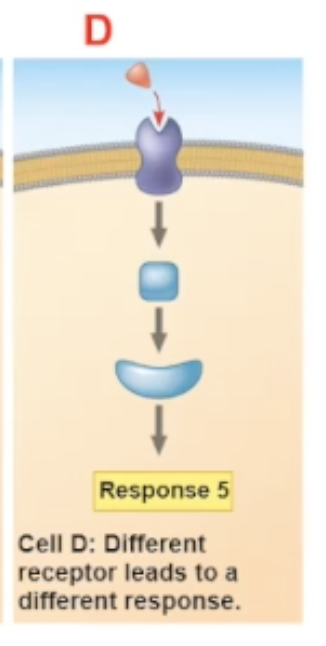
acetycholine binding with different cells
A. heart pacemaker cell- binds to G protein receptor cell. Upon binding it decreases the rate of firing
B. salivary gland cell- binds to G protein receptor cell. Upon binding, the cell secretes saliva
C. skeletal muscle cell- binds to an ion channel-coupled receptor. upon binding, it allows for contraction of the muscle
epinephrin binding to different cells
A. liver cell- binds to its beta receptor. upon binding breaks down glycogen and releases glucose
B. skeletal muscle blood vessel- binds to beta receptor. Upon binding, the vessel dilates
epinephrin binding to same cell different receptors
Receptors in the blood vessel
A. Beta receptor- upon binding, the vessel dilates
B. Alpha receptor- upon binding, the vessel contracts
Signal for apoptosis (example)
for soil worm caenorhabditis elegans
apoptosis is triggered by signals that activate a cascade of “suicide” proteins
when death signal is received, an apoptosis inhibiting protein (Ced-9) is inactivating allowing for the caspase “suicide” proteins to promote apoptosis
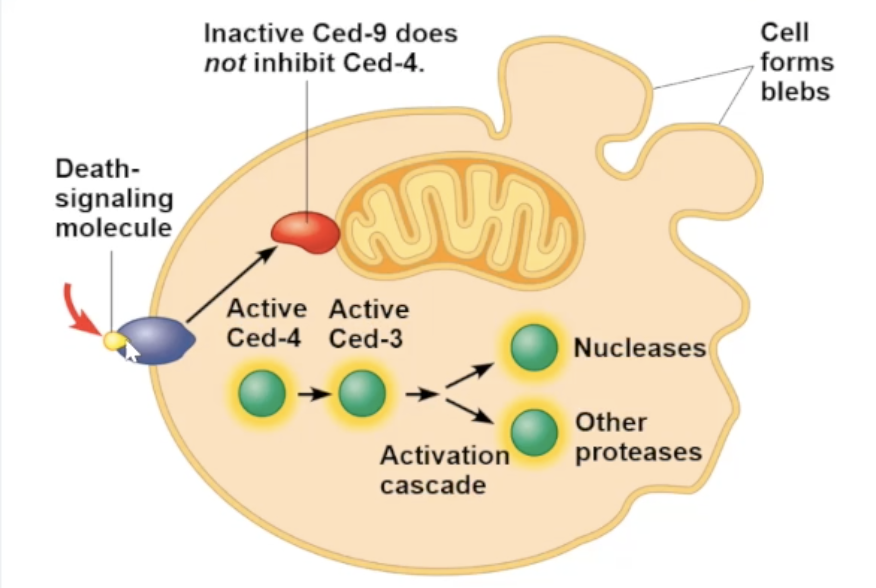
Ced-9 protein
protein inhibiting apoptosis. usually activated unless inactivated
Capases
proteins that carry out apoptosis
Apoptosis pathways and signals triggering them
humans have several different pathways to carry out apoptosis
apoptosis can be triggered by signals outside the cell or inside
internal signals include- DNA damage, protein misfolding
apoptosis steps
initial- sudden release of cytochrome c from the mitochondria into cytosol
second- the lipid asymetry of plasma membrane breaks down (phosphatital serine is found in the cytosol, but during apoptosis it is found outside the cell and other cells such as macrophages can consume the content)
final- the plasma membrane becomes permeable to small molecules