Chapter 22 Organic Compounds
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
34 Terms
What are chloroalkanes?
• Chloroalkanes are compounds in which one or more of the hydrogen atoms in an alkane molecule have been replaced by chlorine atoms

Boiling point - chloroalkane
Liquid at room temperature
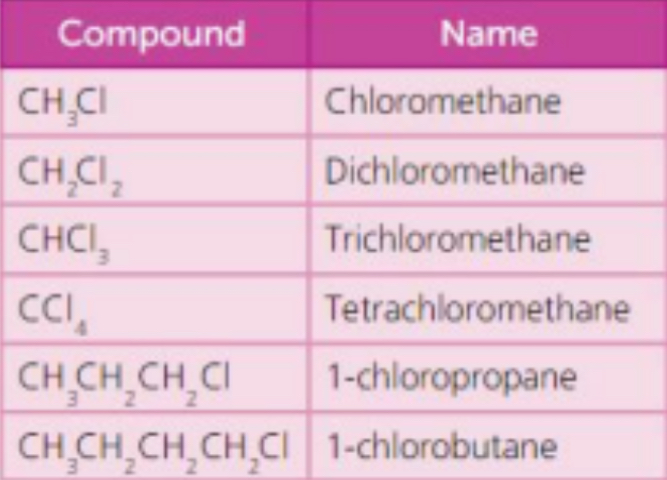
Solubility - chloroalkane
Little Polarity.
Not soluble in water but soluble in non-polar cyclohexane
Give two applications of the chloroalkanes
a) Solvents for non-polar substances – used in dry cleaning
b) Dichloromethane is used in paint stripper
What is a functional group?
• A functional group is an atom or group of atoms responsible for the characteristic properties of an organic compound
1) THE ALCOHOLS
• General Formula: R – OH / CnH2n+2O
• end in “anol”
• Functional group is hydroxyl group (- OH group)
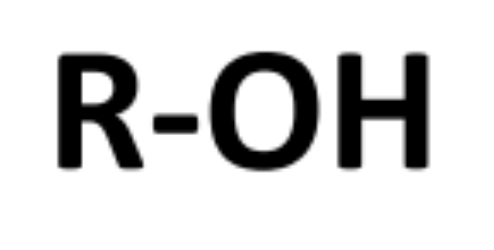
Boiling point - alcohols
Liquid at room temperature. High boiling points than corresponding alkanes due to the presence hydrogen bonding
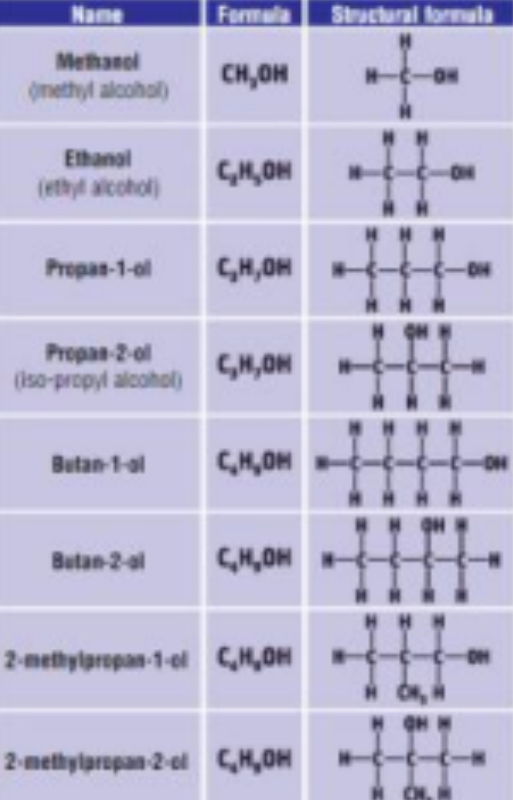
Solubility - alcohols
Short chain carbons (C1, C2 and C3) soluble in water. Longer chain, contain non-polar alkyl which isn't soluble in water.
Give three applications of alcohols
1. Ethanol – used in alcoholic drinks/perfumes/paints
2. Methanol and ethanol are added to petrol as oxygenates to increase octane number
3. Methanol and ethanol are used in methylated spirits – as a solvent and a fuel
How is ethanol produced for its use in alcoholic drinks?
• A process known as yeast fermentation
(Methanol is toxic and is purposely added to industrial alcohol to prevent people drinking it)
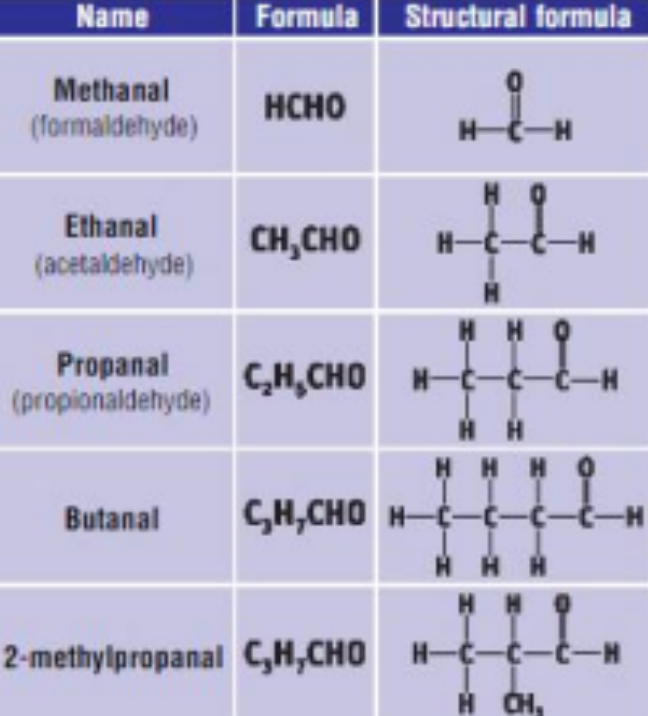
2) THE ALDEHYDES
• General Formula: R – CHO / CnH2nO
• End in “anal”
• Functional group is - CHO group
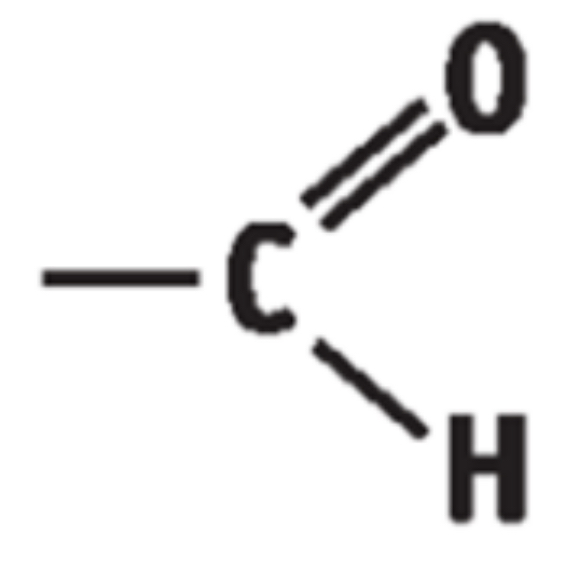
Boiling points - aldehydes
C=0 is polar so dipole-dipole forces exist making boiling points higher than corresponding alkanes but lower than alcohols.
Solubility - aldehydes
Short chains are soluble in water due to hydrogen bonding between the O of C=0 and H of H20.
Solubility decreases with chain length.
Give two applications of aldehydes
1. Formaldehyde (methanal in water) is used to preserve biological specimens
2. Benzaldehyde is found in almond kernels and is used as a flavouring agent in cooking
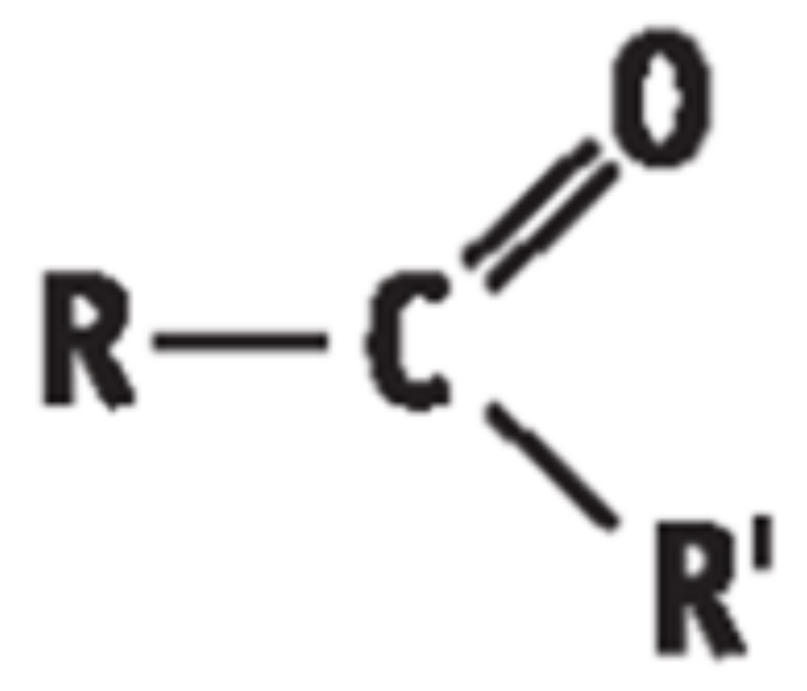
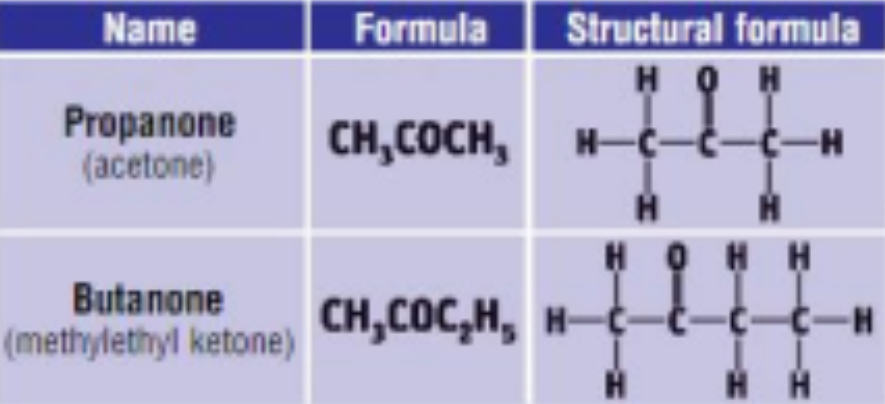
3) THE KETONES
• General Formula: R – CO – R’ / CnH2nO
• End in “anone”
• Functional group is carbonyl group (- CO group) attached to a hydrocarbon portion on either side
Boiling point - ketones
Carbonyl group (>C=0) is polar. Dipole-dipole forces exist so boiling temps are higher than corresponding alkanes.
Solubility - ketones
Short chains are soluble in water due to hydrogen bonding between the O of C=0 and H of H20.
Solubility decreases with chain length.
Give an application of a ketone
• Widely used as solvents – Propanone (acetone) used in nail varnish remover
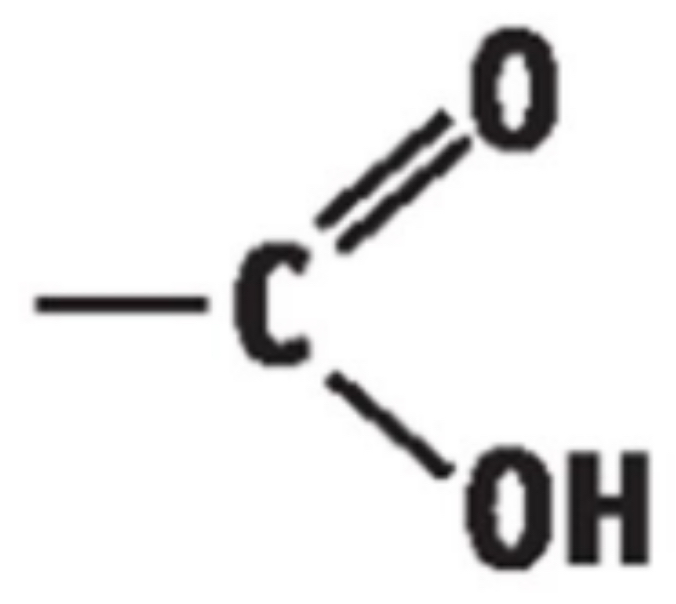
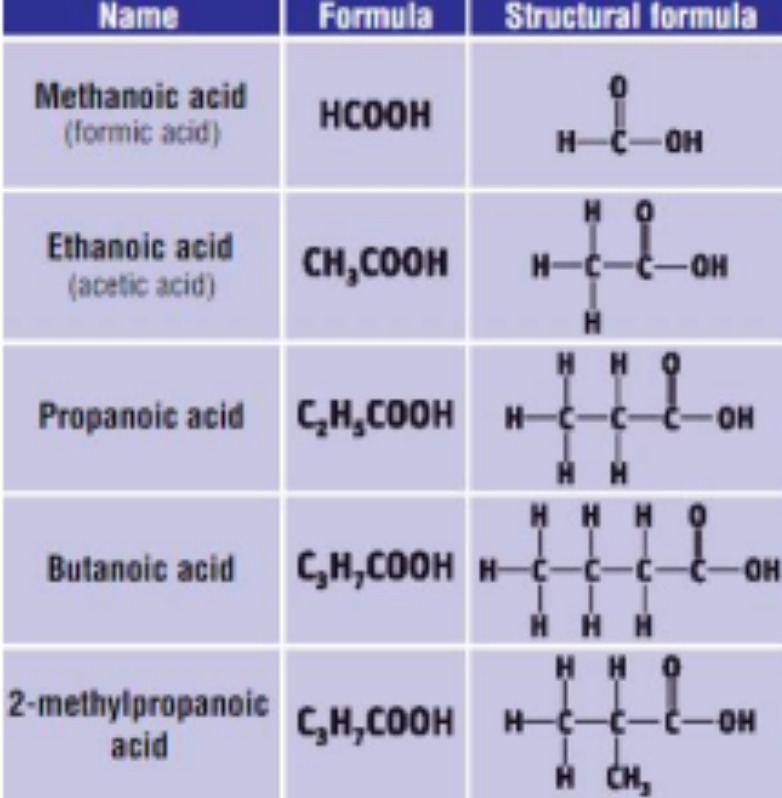
4) THE CARBOXYLIC ACIDS
• General Formula: R – COOH / CnH2nO2
• End in “anoic acid”
• Functional group is carboxyl group/carboxylic acid group
Boiling point - carboxylic acid
Hydrogen bonding exists between molecules so high boiling points. (higher than alcohols)
Solubility - carboxylic acid
Short chains are soluble in water due to hydrogen bonding between the carboxylic acid group and water. Solubility decreases with chain length.
Give four applications of carboxylic acids
1. Ethanoic acid (acetic acid) is the acid in vinegar
2. Ethanoic acid is used to make cellulose acetate - used in varnishes and lacquers
3. Propanoic acid, benzoic acid and its salts are used in food preservation
4. Methanoic acid (formic acid) is found in nettle/ant stings
5 ) THE ESTERS
• General Formula: R – COO – R’ / CnH2nO2
• End in “anoate”
• Functional group is - COOC
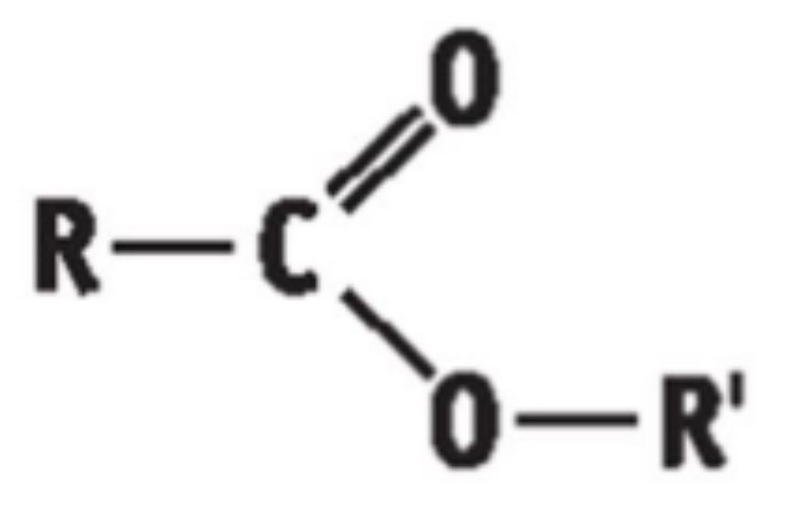
Boiling point - esters
Low boiling points. No hydrogen bonds formed between themselves
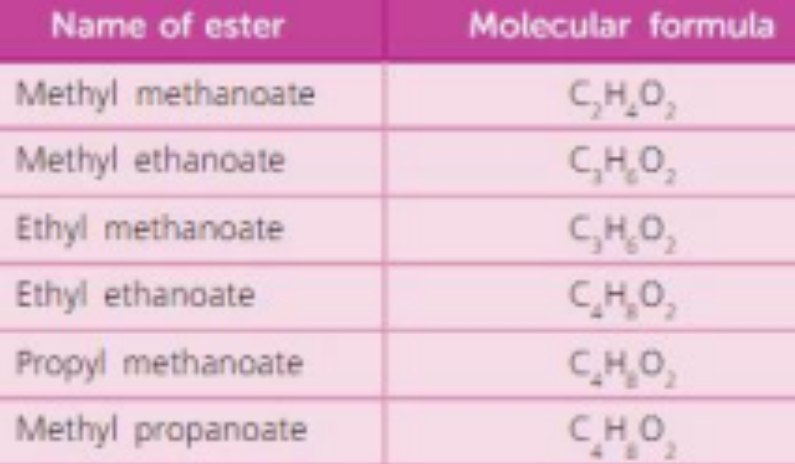
Solubility - esters
As expected, short chains are soluble in water.
Solubility decreases with length.
Give four uses of esters
1. Fats and oils are naturally occurring esters used to make soap
2. Esters are naturally found in fruits (apples, oranges, pears, bananas strawberries)
and are used in fruit flavourings
3. Used in perfumes and cosmetics
4. Ethyl ethanoate is used as a solvent in glue
Responsible for the smell that comes from flowers and fruits.
Boiling points
1. Carboxylic acids
• Highest boiling points of all the organic compounds
- first four members are liquids at room temperature
2. Alcohols
• Lower boiling points than carboxylic acids
• Higher boiling points than aldehydes, ketones, esters, alkanes, alkenes, alkynes
- first four members are liquids at room temperature
3. Aldehydes, Ketones, Esters
• Lower boiling point than carboxylic acids and alcohols
• Higher boiling point than alkanes, alkenes, alkynes
(- Aldehydes: methanal is a gas and ethanal is a volatile liquid at room temperature
- Ketones: propanone and butanone are volatile liquids at room temperature
- Esters: lower members are volatile liquids)
4. Alkanes, Alkenes, Alkynes
• Lowest boiling points of all the organic compounds
- first four members (C1- C4) are gases at room temperature
• Van der waals forces form between the molecules - due to the molecules being completely
non-polar
• Van der waals forces are the weakest intermolecular force
• Boiling point increases as molecular mass increases due to stronger intermolecular forces
forming
Solubility
1. Alkanes, Alkenes, Alkynes
• Completely non-polar hydrocarbons
2. Carboxylic acids, Alcohols, Aldehydes, Ketones and Esters
• Contain both a non-polar hydrocarbon portion and a polar portion (Hydroxyl group or carbonyl group)
Distinguish between aliphatic compounds and aromatic compounds
Aliphatic compounds: Organic compounds where carbon atoms formm open chains or closed rings but does not contain a benzene ring in their structure
Aromatic compounds: Organic compounds containing a benzene ring in their structure
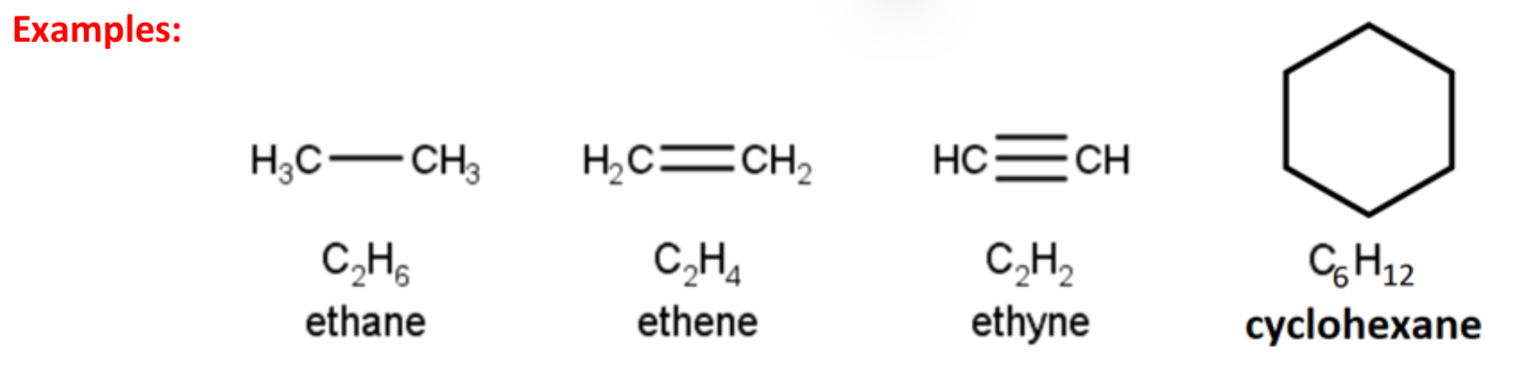
Describe the bonding in benzene?
• Six carbon-hydrogen single bonds are formed by sigma bonds – 12 sigma electrons fixed
• Six carbon-carbon ‘single bonds’ are formed by sigma bonds – 12 sigma electrons fixed
• Three carbon-carbon ‘double bonds’ are formed by 6 pi electrons
• The 6 pi electrons are delocalised and are free to move around the benzene ring (as if position of the double bonds constantly changes)
• Because of this delocalised structure, benzene is more correctly described has having no single bonds or double bonds between carbons, but all carbon-carbon bonds are an identical intermediate between single and double bonds
What are delocalised electrons?
• Delocalised electrons are freely moving and are shared between MORE THAN TWO ATOMS
Explain how the Kekule structure correctly describes benzene’s structure
• The Kekule structure correctly describes:
a) The number of carbon-hydrogen sigma electrons – six single bonds with 2 electrons each = 12 sigma electrons
b) The number of carbon-carbon sigma electrons – six ‘single bonds’ with 2 electrons each = 12 sigma electrons
c) The number of carbon-carbon pi electrons – three ‘double bonds’ with 2 pi electrons each = 6 pi electrons
Q: Explain how the Kekule structure DOES NOT correctly describe benzene’s structure
• The Kekule structure does not correctly describe the distribution of the pi electrons
• The Kekule structure has the pi electrons localised to three double bonds but the electrons are delocalised
• Benzene has no single bonds or double bonds between carbons, but all carbon-carbon bonds are an identical intermediate between single and double bonds
Give two properties of benzene that result from its unusual bonding structure
1) Lack of reactivity – Benzene is very stable/unreactive
Experimental evidence for this: Benzene will not decolourise red bromine water or purple acidified potassium manganate (VII) as normal unsaturated compounds (not undergo an addition reaction)
- it is NOT unsaturated i.e. it has no double bonds - all carbon-carbon bonds are an identical intermediate between double and single bonds
2) Unusual bond length - Single bonds in general are longer than double bonds but all of benzene’s carbon-carbon bonds are the same length
Experimental evidence for this: The bond lengths in benzene are measured to be in between single bond and double bond length
What is the average number of electrons shared between any two adjacent carbon atoms in a molecule of (i) cyclohexane, (ii) benzene?
(i) Cyclohexane = 2 electrons - only single bonds in cyclohexane
(ii) Benzene = 3 electrons - 2 sigma electrons always shared + 2 delocalised electrons ‘sometimes’ shared
Common aromatic compounds
Dyestuffs - Chrysoidine
• Detergents - Sodium dodecylbenzenesulfonate
• Herbicides – Diuron
• Pharmaceuticals– Paracetamol, Asprin, Ibuprofen, Morphine, Penicillin
• Narcotics – Cocaine, Heroin
• Acid-Base Indicators – Methyl Orange, Phenolphthalein
• Food preservatives - Benzoic acid and its salts (sodium benzoate)