AICE Marine Science: Topic 1 Water
1/47
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
48 Terms
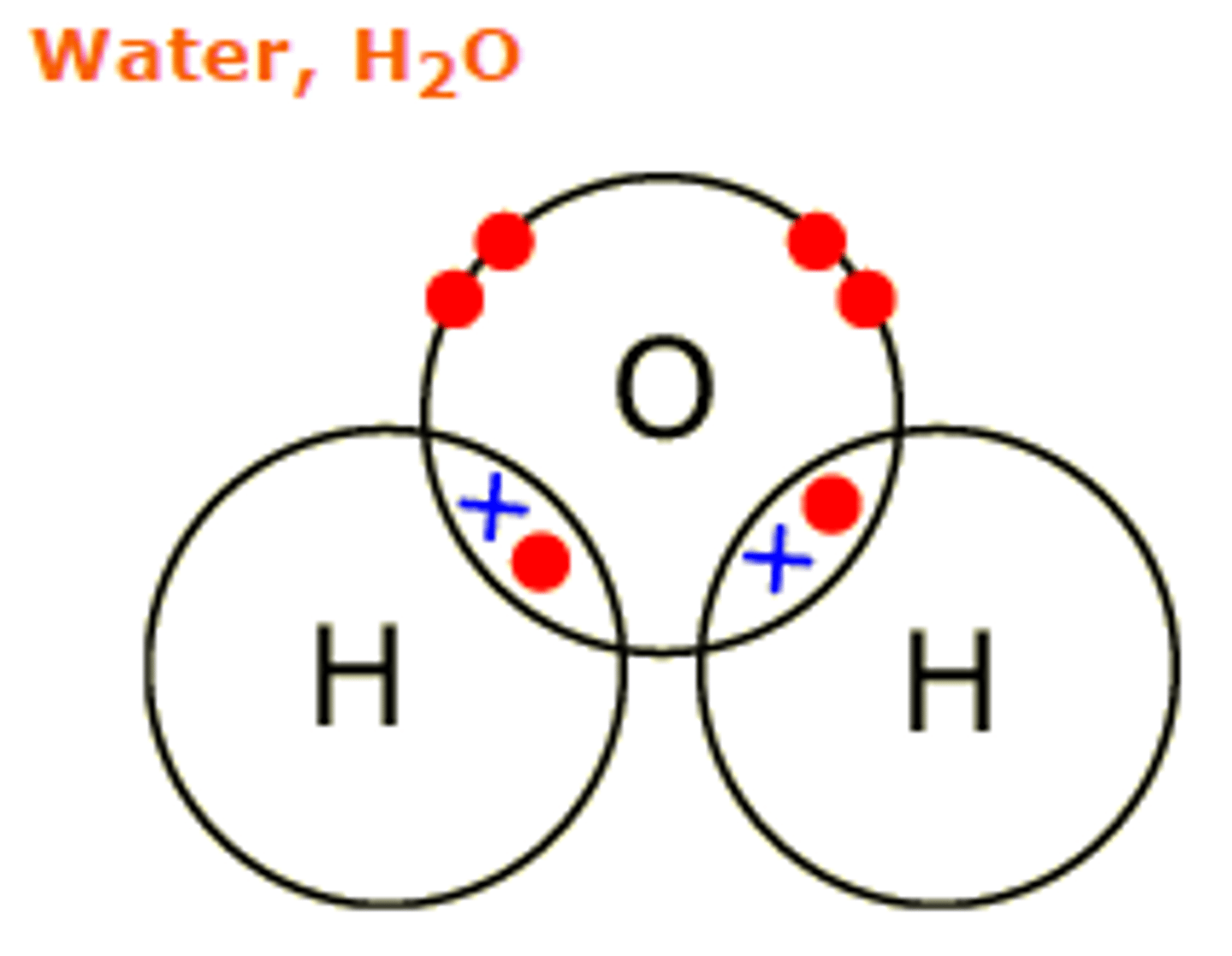
one oxygen (-) and two hydrogen (+)
Draw a water molecule
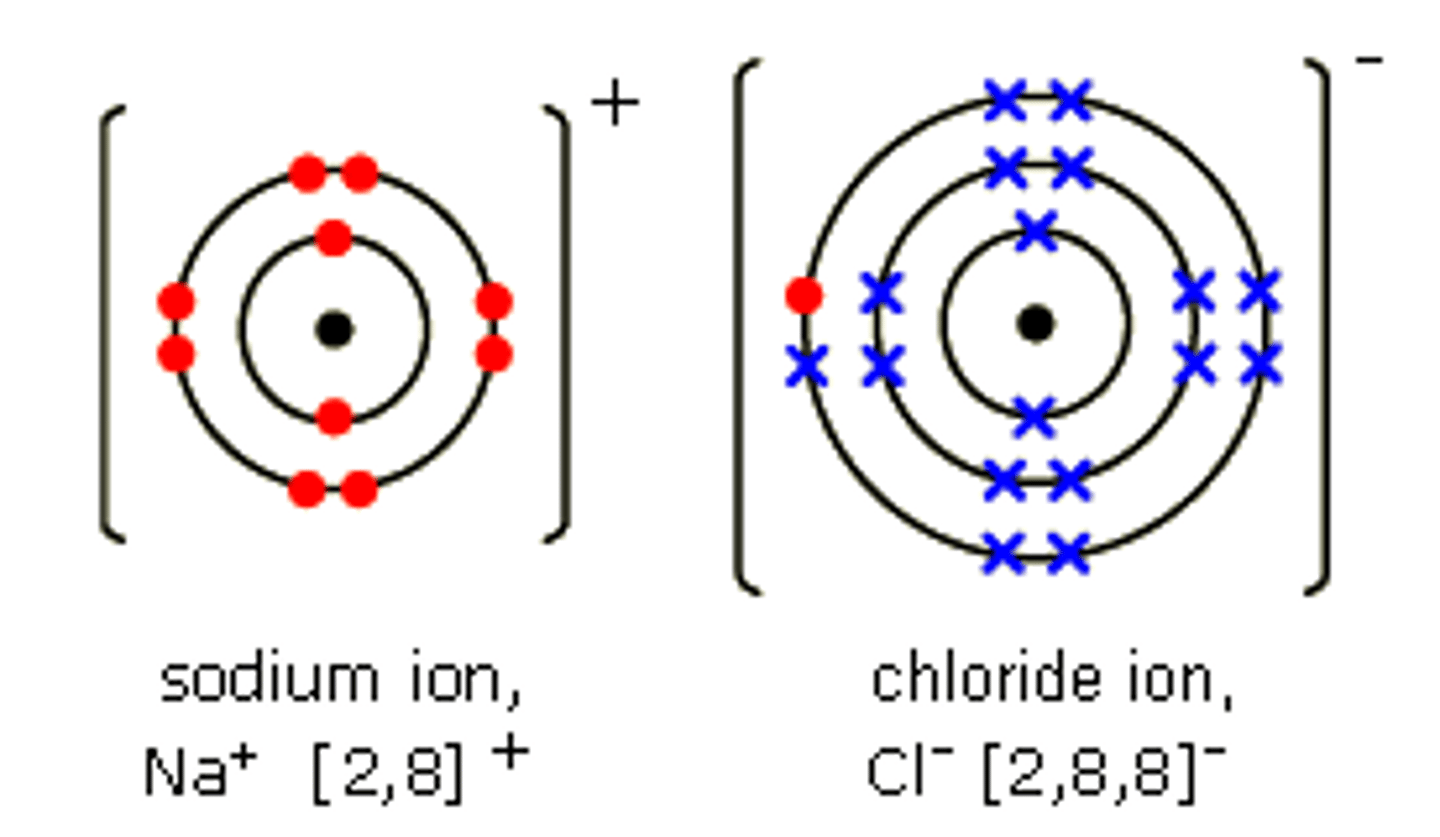
chlorine atom gains one electron ; sodium atom loses one electron ;to fill / complete outer (valency) shell ;
opposite charges (of ions) attract (to form lattice) ;
Explain the formation of sodium chloride (NaCl)
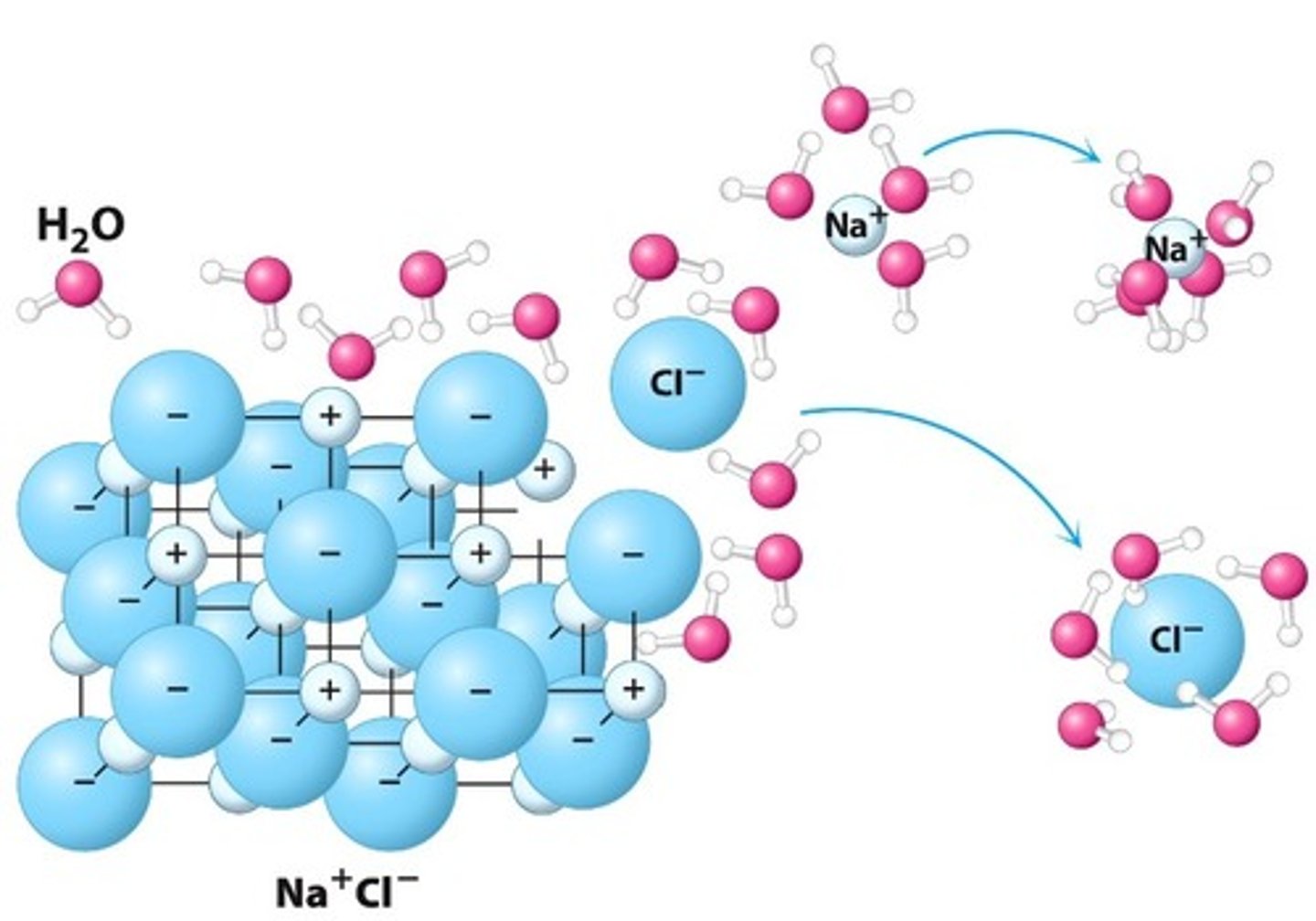
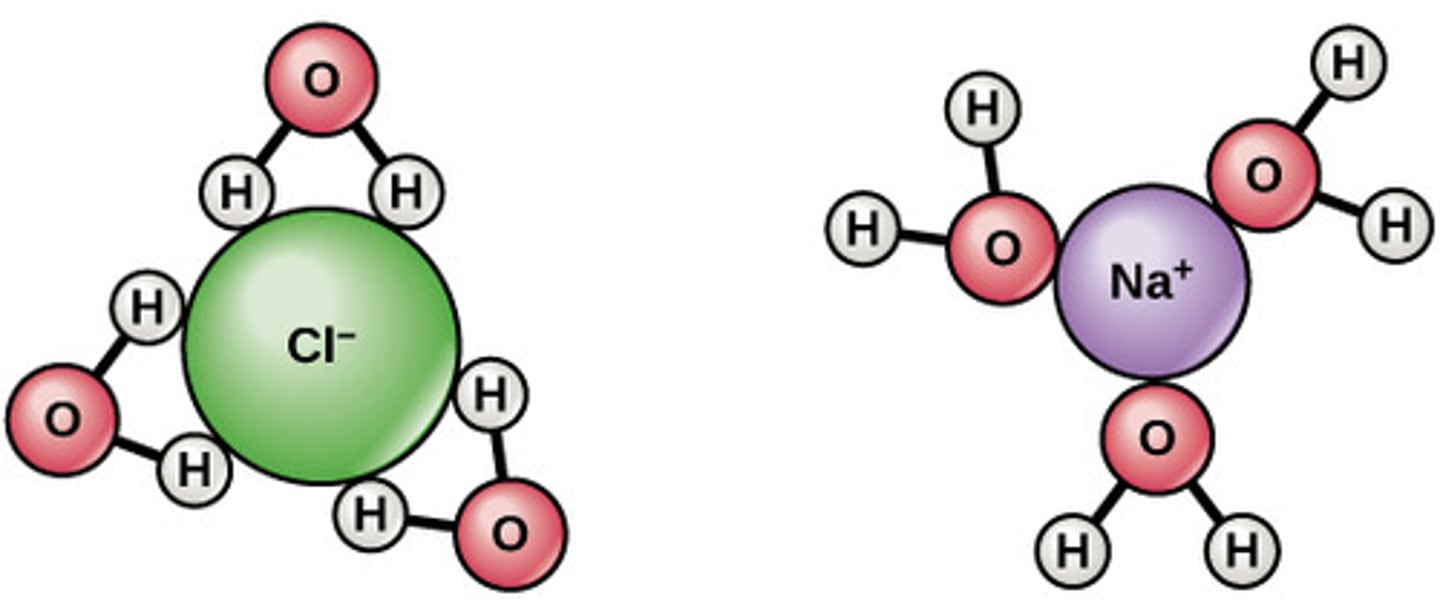
Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a homogeneous solution
How sodium chloride dissolves in water
as depth increases, salinity increases
explain the relationship between depth and salinity
runoff, evaporation, precipitation, ice formation, and ice melting.
Factors affecting salinity

decrease the density of seawater
effect of high temperature on density on seawater
increases the density of seawater
effect of higher salinity on density of seawater
amount of oxygen dissolved in water
Dissolved Oxygen (DO)
Partial charges on water molecules allow water to interact with charged ions and covalent substances
Capable of forming bonds with unusually large number of substancesbstances d
Why is water the universal solvent?
as water heats up, it increases the kinetic energy making it easier for water molecules to break the ionic bonds and surround the positive and negative ions that are left.
Impact of higher temperature of seawater on the rate of dissolution of salts increases.
0 degrees Celsius
freezing point of water
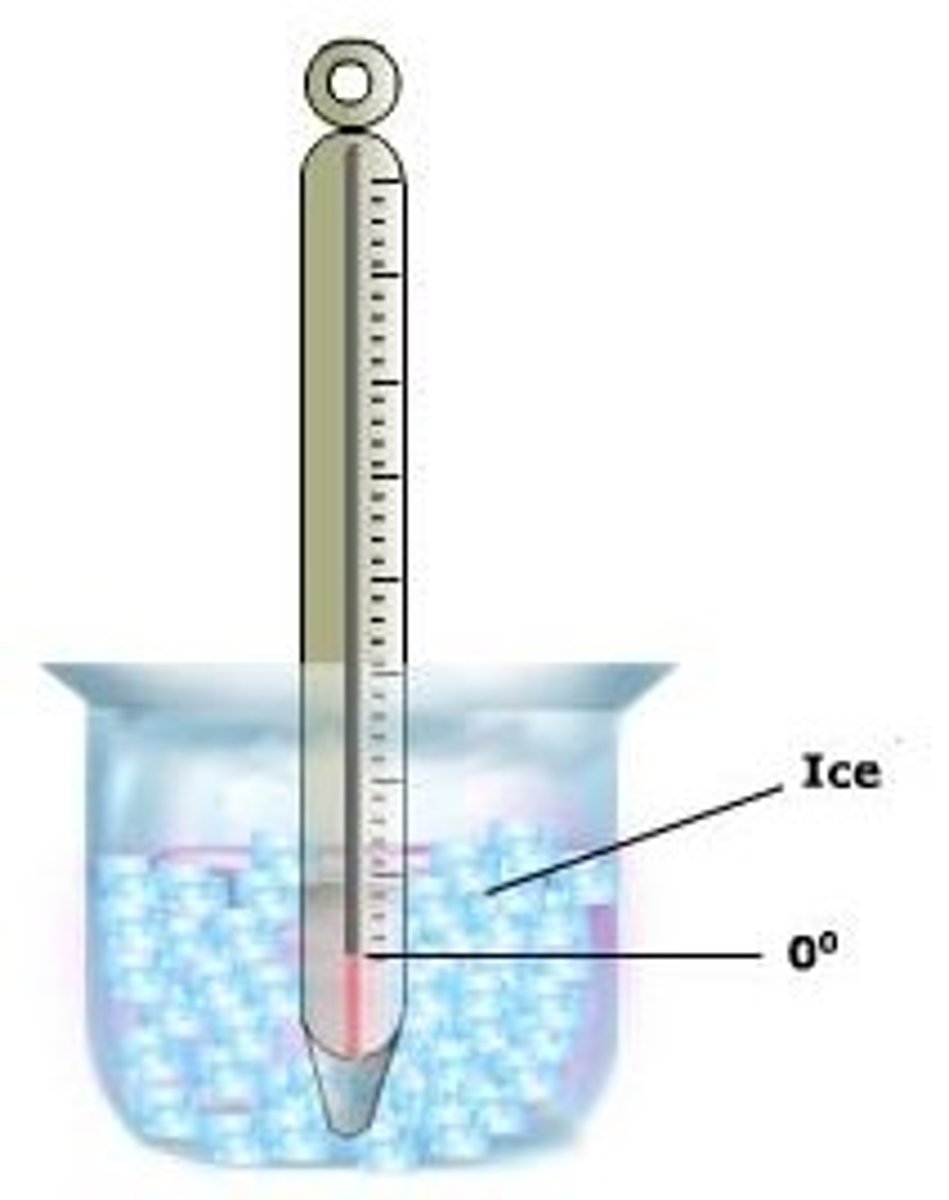
The higher the concentration of salt, the lower the freezing point drops.
Impact of salinity on freezing point
Hydrogen bonds (H-bonds) reduce density
Water molecule movement slows
H-bonds between water molecules strengthen
Stronger H bonds able to keep water molecules at perfectly symmetrical distance from each other forming crystal lattice
Fewer water molecules able to fit in same volume
Density is reduced in the solid → ice
Why does Ice Float?
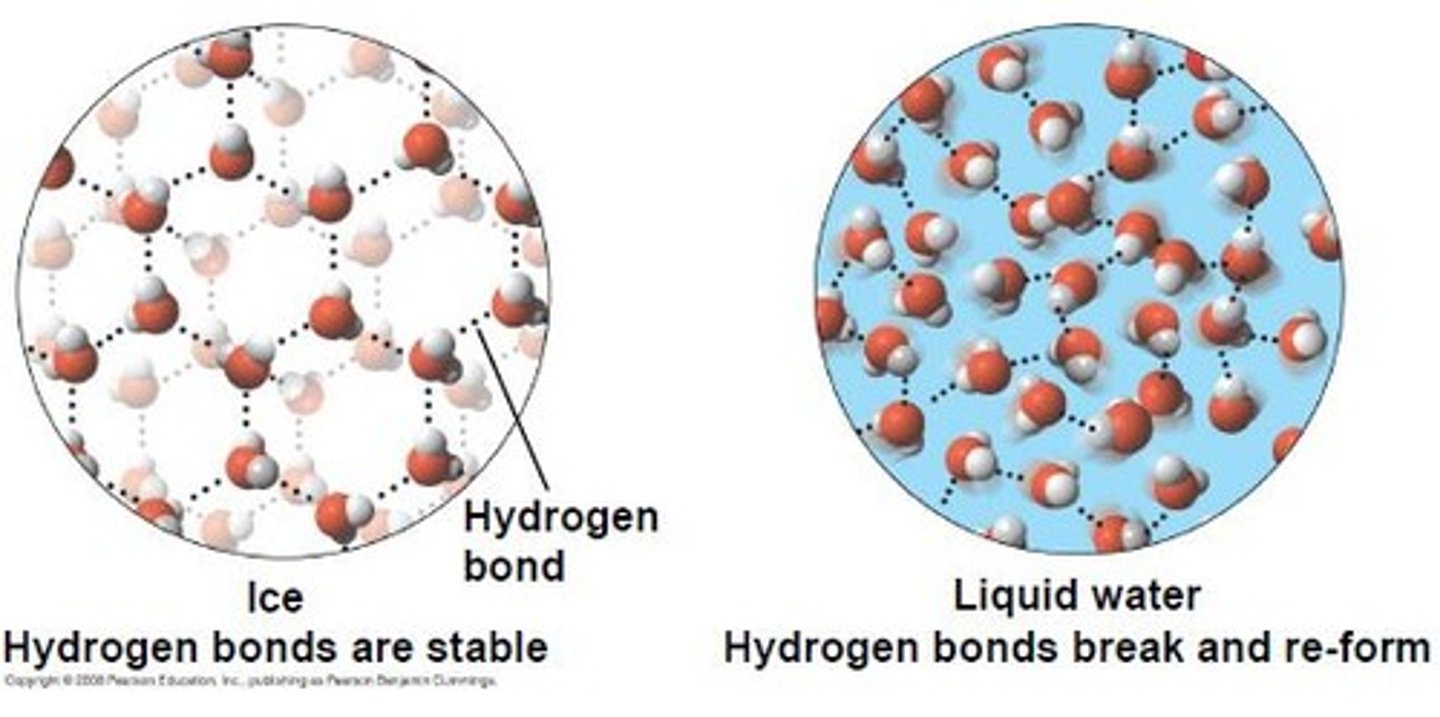
Due to number of hydrogen bonds in liquid water
Allows water to act as temperature buffer and climate regulator globally
Size of oceans allows them to hold a tremendous amount of heat energy before the water will change temperature
Keeps coastal climates mild, impacts rain patterns, influences wind
Why does water has one of the highest specific heat capacities
when a body of water has a salinity level greater than 40 ppt
Hypersaline

evaporation, formation of sea ice
What increases salinity?
precipitation, runoff from land, icebergs melting, sea ice melting
What decreases salinity?

Most hypersaline environment on Earth
Don Juan Pond in Antarctica
numeric value expressing the acidity or alkalinity of a solution
Symbol for 'potential of hydrogen'
Abiotic factor within ecosystems
Particularly important for aquatic ecosystems like the ocean
Used to measure the concentration of hydrogen ions in a liquid
pH
A substance that increases the hydrogen ion concentration of a solution.
Acid
a compound that produces hydroxide ions in solution
Base
Ratio of H+ : OH-
determines the pH of a solution
pH meter or data-logging pH probe.
limitations: probe must be cleaned between each reading and reading must be taken from same depth
How to measure pH quantitively?
8.1
What is the current pH of the ocean?
nitrogen, oxygen, carbon dioxide
Major gases in the atmosphere and ocean
irregular changes in the speed and direction of fluid movement due to wave action causes mixing at the surface of the ocean
Turbulence
the concentration within seawater increases
As concentration of gas in atmosphere increases
high due to due to ability to form carbonic acid when mixed with water
solubility of carbon dioxide
low Does not chemically combine with water molecules
solubility of oxygen
The temperature is inversely proportional to the solubility of the gas in a liquid, hence for all gases, as the temperature increases, solubility decreases accordingly.
Explain the solubility of gases in relation to water temperature.
Less molecule movement
Better able to dissolve more gases (higher solubility)
more dense
greater depths
higher salinity
cold water
More molecule movement
Easier for dissolved gas molecules to leave water and enter atmosphere
Gas molecules are less soluble in warm water
surface ocean
absorbs incoming radiation (warmer)
less dense
warm water
solubility increases due to
why?
Higher pressure → higher concentration of gases → gases forced into the water (increases solubility)
As atmospheric pressure increases
solubility decreases
why?
Lower pressure → lower concentration of gases in atmosphere → more gases escape the seawater (decreases solubility)
As atmospheric pressure decreases
Gases dissolve better in water with low to no salinity
Because fewer solutes are taking up the spaces available to the gases
Allows for more interaction between water molecules and gases
How does salinity impact solubility?
freshwater>estuary> open ocean
Where are gases more soluble?
a layer of water in which the salinity changes rapidly with changes in depth
Halocline
the layer within the ocean where the concentration of dissolved oxygen is at its lowest, typically found between 100 and 1000 m deep
oxygen minimum layer
Greatest DO concentration in top 100m
at what depth is the DO the most saturated?
Turbulence / mixing of water
Photosynthesis by producers (i.e. algae, phytoplankton)
oxygen is a by-product
Why does the surface have the highest DO?
the zone that receives enough light to allow photosynthesis to occur
photic zone (ocean)
necessary for photosynthesis
Why is CO2 important?
Used by all living organisms for respiration
Why is O2 important?
Transformed into ammonia (NH3) by bacteria
Makes nitrogen usable for other organisms
Needed for amino acids & proteins, DNA & RNA
Why is nitrogen gas important?
mass/volume
Density
kg/m³
unit of density
temperature changes drastically
In tropical seas the thermocline is steep as water temperature at the surface can be 25°C or higher, while water at 2000m and greater is at 1°C
In polar seas there is less likely to be a thermocline at all
Temperature gradient is subtle
Little difference between temperature at surface and in deep waters
Thermocline in tropical waters (low latitudes)
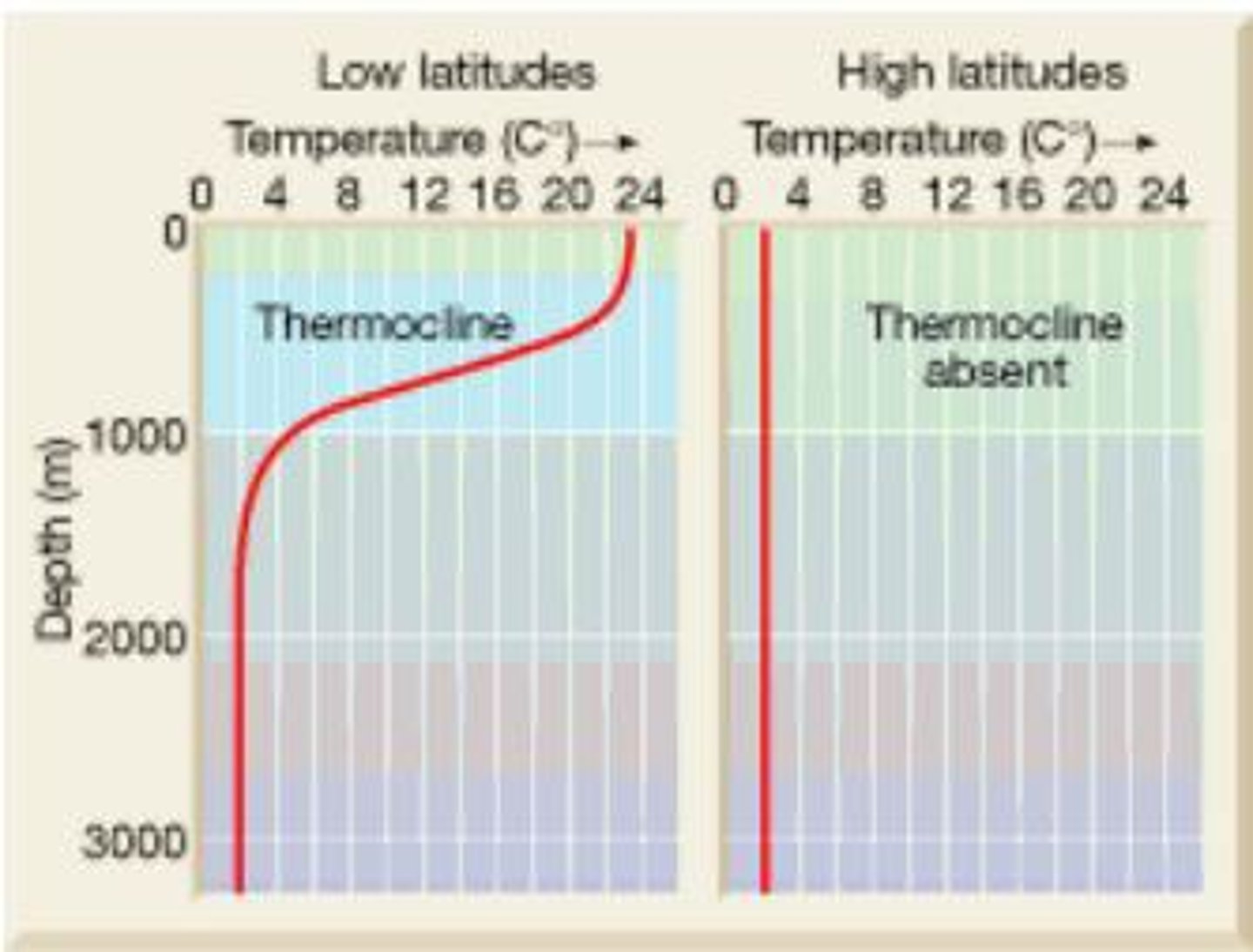
In polar seas there is less likely to be a thermocline at all
Temperature gradient is subtle
Little difference between temperature at surface and in deep waters
thermocline in polar regions (high latitude)