Cluster-1: Immunodeficiency & Immunomodulation
1/48
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
49 Terms
What is complement immune system
A complex system of proteins in the blood that enhances the body's ability to fight infections by promoting inflammation, opsonization, and cell lysis.
What are complement deficiencies
a group of immunodeficiency disorders resulting from the impaired function or production of complement proteins, leading to increased susceptibility to infections.
What are the three pathways of complement
Classical pathway
Alternative pathway
Lectin pathway
These pathways work together to trigger the complement cascade and enhance immune responses.
What is the classical pathway process explained
How it's triggered:
Activated when antibodies (IgG or IgM) bind to a pathogen (e.g., bacteria, viruses).
The C1 complex (C1q, C1r, C1s) recognizes these antibody-pathogen complexes and starts the cascade.
Key features:
Requires adaptive immunity (antibodies).
Helps phagocytes recognize pathogens by marking them (opsonization).
Creates membrane attack complexes (MACs) that punch holes in microbes to kill them.
What is the alternative pathway process explained
How it's triggered:
Always active at low levels because complement protein C3 spontaneously breaks down into C3b.
If C3b binds to a pathogen, it stabilizes and starts the pathway.
A protein called Factor B helps amplify the response.
Key features:
First line of defense since it does not require antibodies or sugar recognition.
Works continuously to monitor for invaders.
Can also amplify the other pathways.
What is the lectin pathway process explained
How it's triggered:
Activated when mannose-binding lectin (MBL) or ficolins recognize sugars (mannose) on microbial surfaces.
MBL then activates MASPs (MBL-associated serine proteases), which continue the complement cascade.
Key features:
Does not require antibodies, so it acts faster than the classical pathway.
Targets microbes based on sugar patterns, which are different from human cell sugars.
Acts as a bridge between innate and adaptive immunity.
What are membrane-bound regulators purpose
They help control the complement activation process on host cells, preventing damage to own tissues. They regulate the activity of complement proteins on host cell surfaces
What are the three membrane regulators used in the complement system
DAF = Decay Accelerating Factor (dissociates C3 convertase)
MAC-IP = Membrane Attack Complex – Inhibitory Protein (inhibits polymerization of C9)
MCP = Membrane Cofactor of Proteolysis (degrades C4b and C3b)
What are the three diseases studied that are related to complement
Age-related Macular Degeneration
Systemic Lupus Erythematosus
Inflammatory Bowel Diseases
Explain the main pathway that is used in complement deficiency
ALTERNATIVE Pathway
C3b can bind to any –OH or–NH2 groups: either microbial cell or human cell. Binding on to microbial cells leads to complement activation and cell lysis.
However
iC3b is biologically inactive to bind to factor B. Complement cascade cannot proceed on human cells
What is the function of sialic acid and glycosaminoglycans
Sialic acid and glycosaminoglycans play crucial roles in inhibiting complement activation on cell surfaces. facilitate binding of regulators from blood
They help protect cells from complement-mediated lysis by acting as barriers to the binding of complement components.
What are soluble regulators
Soluble regulators are proteins in the bloodstream that modulate the complement system's activity, preventing excessive inflammation and tissue damage.
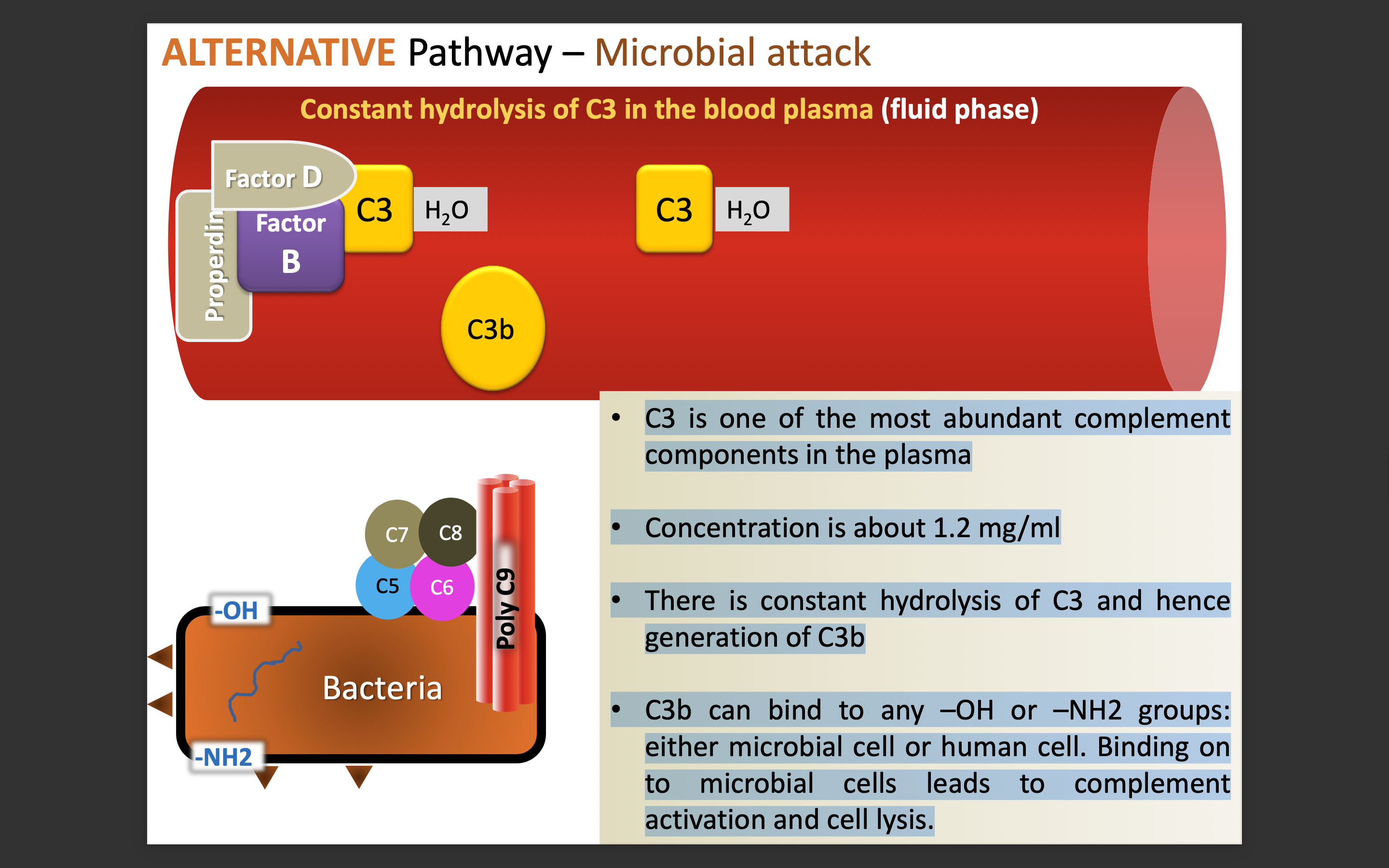
What is the process of microbial attack in alternate pathway
The alternative pathway is activated when C3b binds to microbial surfaces, leading to a cascade that enhances opsonization and lysis of pathogens.
This process bypasses the need for antibodies, directly targeting pathogens for destruction.
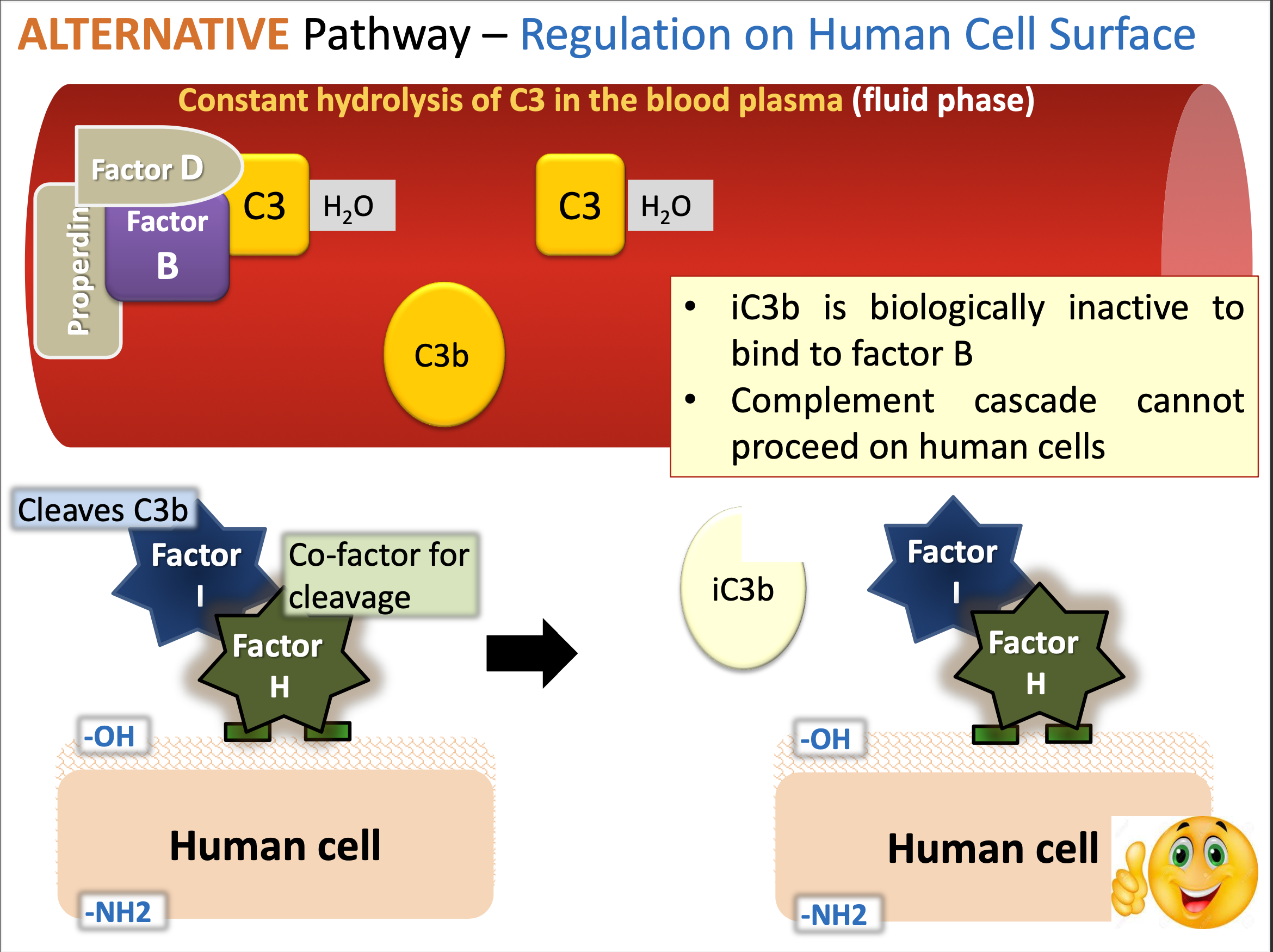
What is the alternate pathway process on regulation on human cell surface.
The alternate pathway regulates complement activation on human cell surfaces by utilizing factors that prevent the enhancement of lysis.
Properdin stabilizes the C3 convertase (C3bBb) on microbial surfaces while regulators like factor H and factor I prevent complement activation on host cells.
What is AMD
Age-related Macular Degeneration (AMD) is a common eye condition that affects the macula, leading to vision loss in older adults. It is characterized by the deterioration of the central portion of the retina.
What polymorphism can occur to affect the factors
Factor H gene polymorphism (Y402H) produces non-functional factor-H protein
Normal: Factor H anchors on to the glycosaminoglycans present on the human cell surface;
This enables factor-I to cleave C3b into iC3b (inactive); This inhibits complement activation.
Mutant fails to bind Glycosaminoglycans on human cells; As a result, C3b is not inactivated
This leads to uncontrolled C3/complement activation and chronic inflammation in the retina
New fragile blood vessels; if left untreated, leads to severe AMD
What factors can contribute to polymorphism in the factor
Smoking
Cholesterol
What is the connection between smoking and complement factors
The paper provided states that “Cigarette Smoke Can Activate the Alternative Pathway of Complement”
It states that smoke can deplete the haemolytic capacity of whole human serum.
The paper mentioned that treatment of purified human C3 with whole smoke solution modifies the C3 molecule
It results in the consumption of hemolytic complement by activation of the alternative pathway as the classical pathway was blocked with Mg/EGTA.
In addition, the paper mentions that smoke-modified C3 has diminished binding to Factor H
ER: What is the connection between cholesterol and complement factors
factor H has a gene that is Y402H variant
This Y402H variant is more likely to increase risk of acquiring AMD
The paper mentioned that it fed mice a high-cholesterol diet and mice with the variant developed AMD-like symptoms
A symptom like:
increased retinal pigmented epithelium (RPE)
increased basal laminar deposits
How do we diagnose the factor gene polymorphism
Sequencing is a method to detect SNPs for factor H
Identify Changes: In the Y402H polymorphism, one specific change occurs:
Normal: The codon for tyrosine (Y) is present.
Variant: The codon changes to produce histidine (H) instead.
By comparing the sequenced DNA to the normal CFH gene, you can see if someone has:
Normal CFH (Y402/Y402): No risk variant.
Heterozygous (Y402/H402): One copy of the risk variant.
Homozygous (H402/H402): Two copies of the risk variant.
This information helps assess the individual's genetic risk for AMD.
What are the treatments for AMD
Anti-VEGF: Monoclonal Antibody against Vascular Endothelial Growth Factor is the current treatment; Aims to prevent growth of new blood vessels
Under development
Compstatin-A: peptide that binds C3; Injected intra-vitreally; forms a gel and slow release of peptide molecules over months
Recombinant factor-H: Reconstitute the serum and inhibit only alternative complement pathway
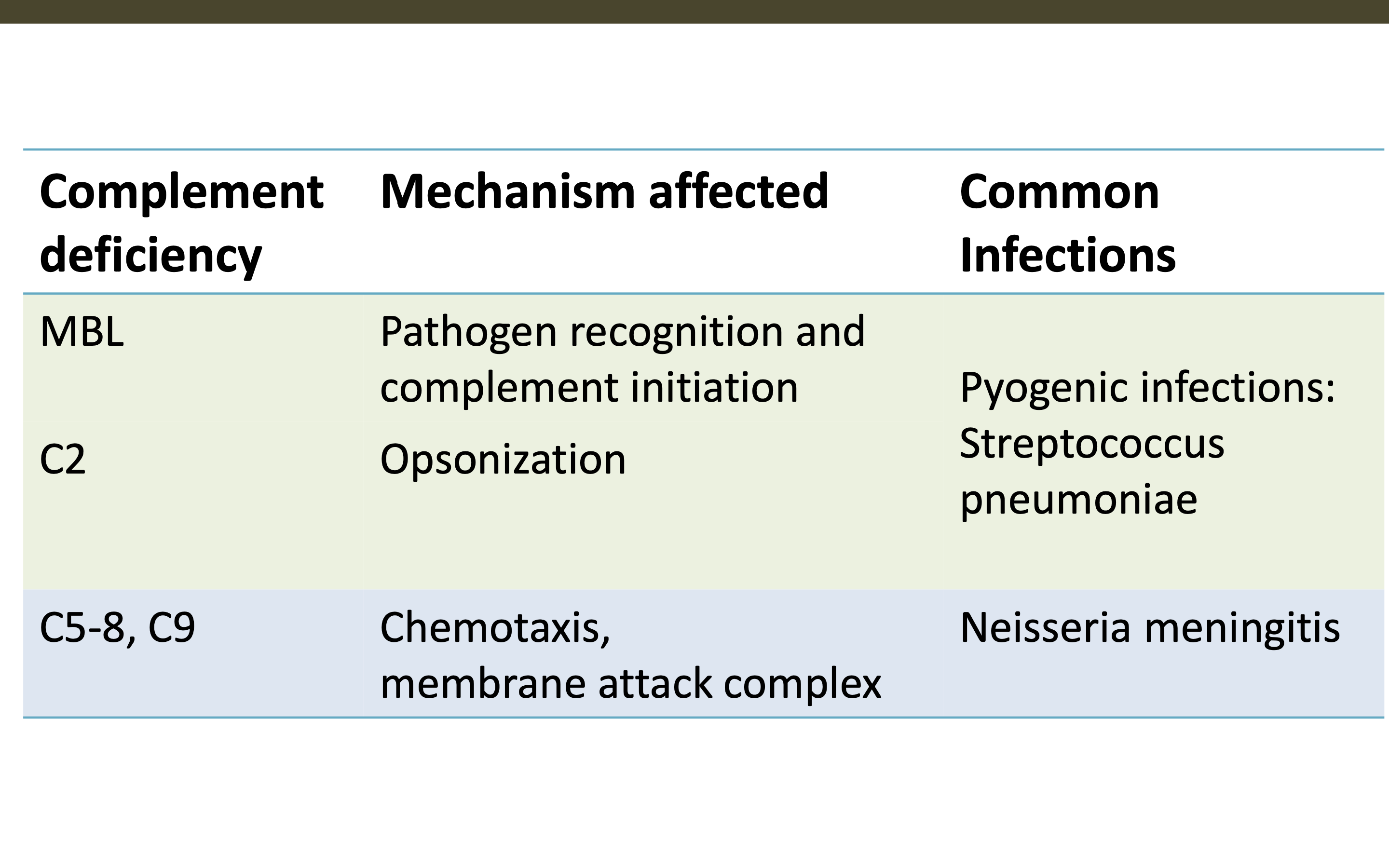
What are the common infections from these complement deficiences and how are their mechanisms affected?
MBL
C2
C5-8, C9
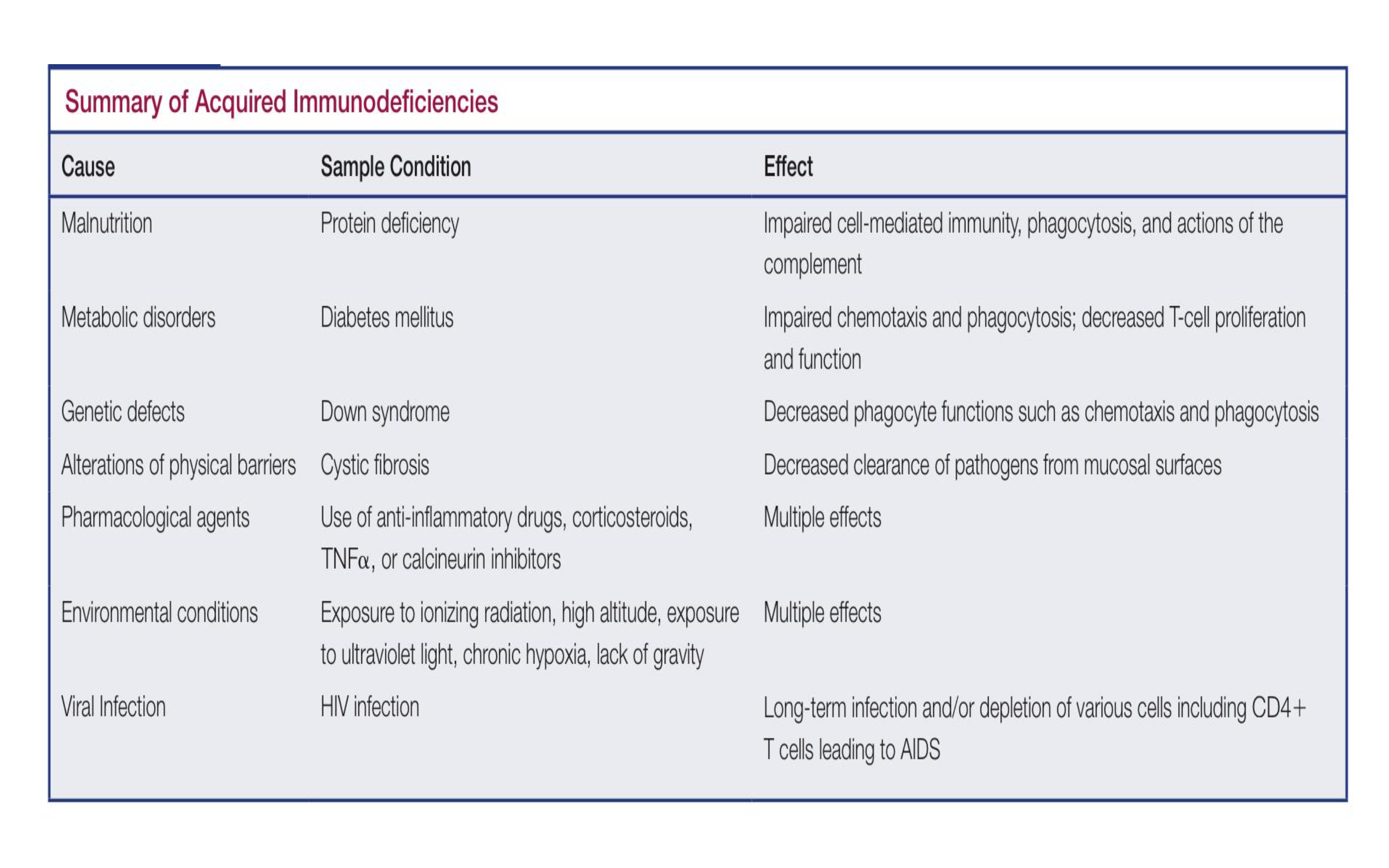
What is acquired immunodeficiency
A condition that occurs when the immune system is weakened, typically due to infections such as HIV, certain medications, or malnutrition, resulting in increased susceptibility to infections and diseases.
What is HIV
Human Immunodeficiency Virus, a virus that attacks the immune system, specifically CD4 T cells, leading to acquired immunodeficiency syndrome (AIDS) if untreated.
Retrovirus
enveloped RNA viruses
uses reverse transcriptase in the conversion of viral RNA to DNA, which is then incorporated into the host genome.
2 types:
HIV-1 (1981, global epidemic)
HIV-2 (West Africa)
Genus Lentivirus, family Retroviridae
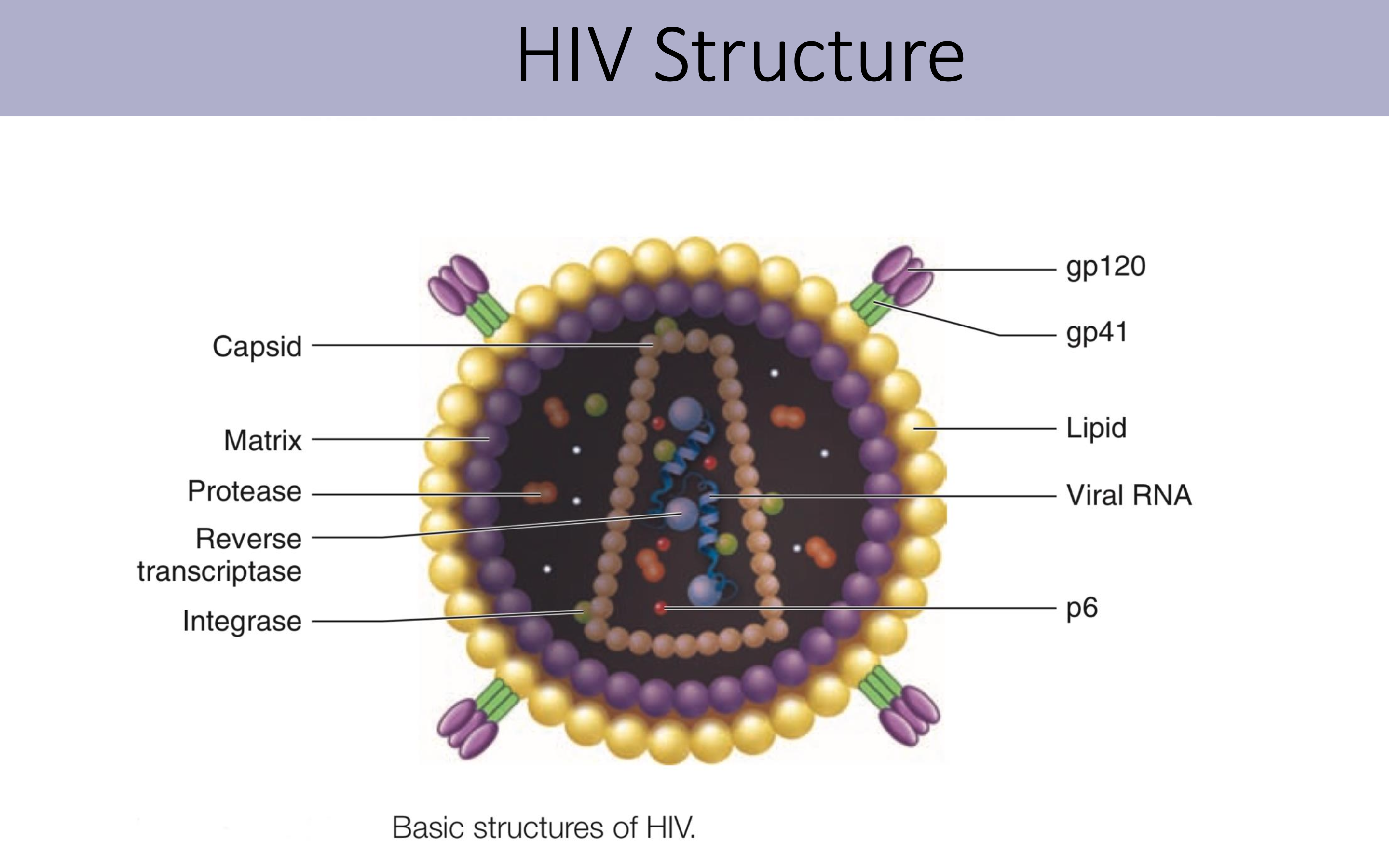
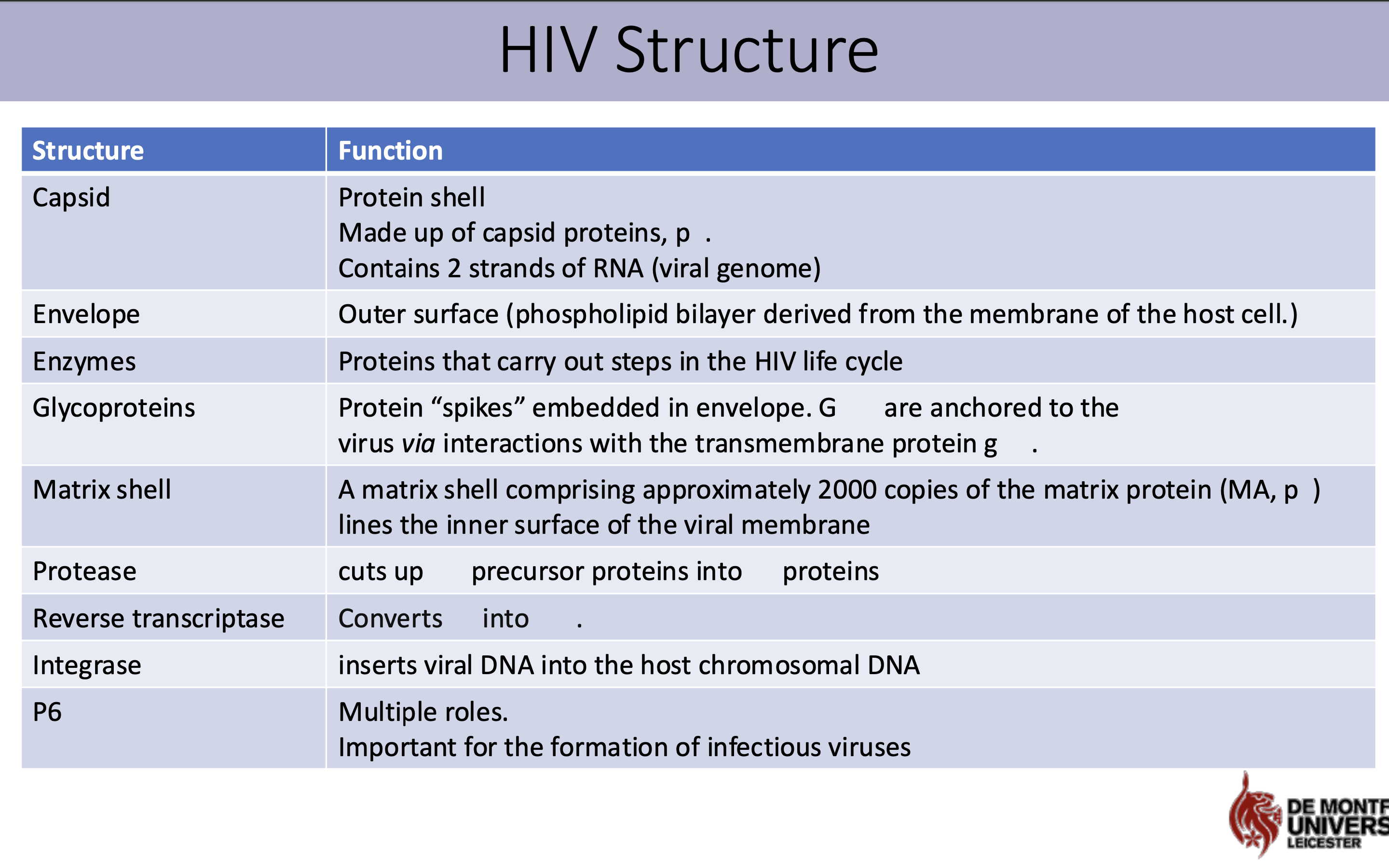
What is the structure and function of HIV virus
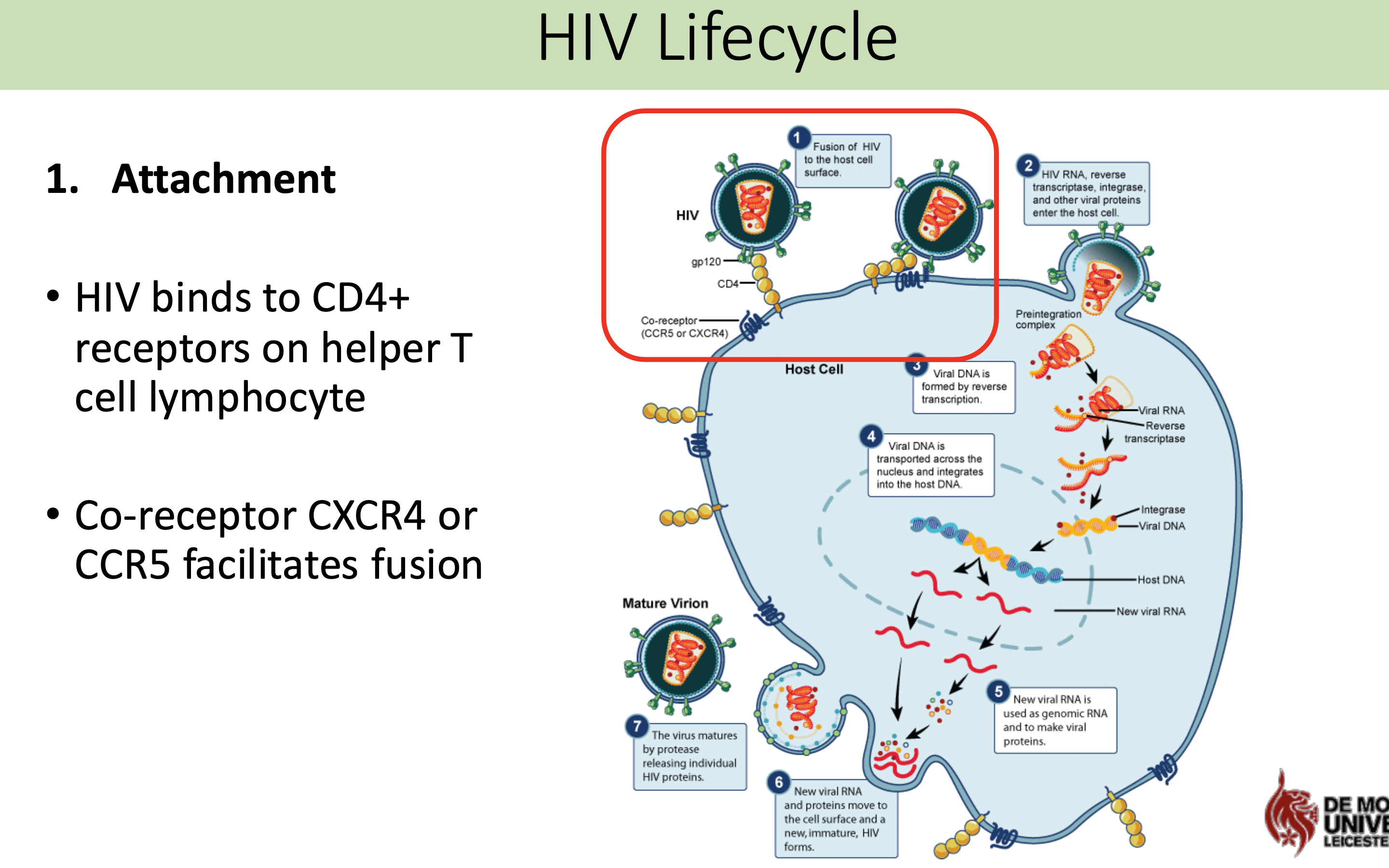
What are the 7 stages of the HIV life cycle
"All Fat Rabbits In Red Are Bouncy"
1. Attachment
2. Fusion
3. Reverse Transcription
4. Integration
5. Replication
6. Assembly
7. Budding
What happens in attachment stage in HIV life cycle
HIV binds to CD4+ receptors on helper T cell lymphocyte
Co-receptor CXCR4 or CCR5 facilitates fusion
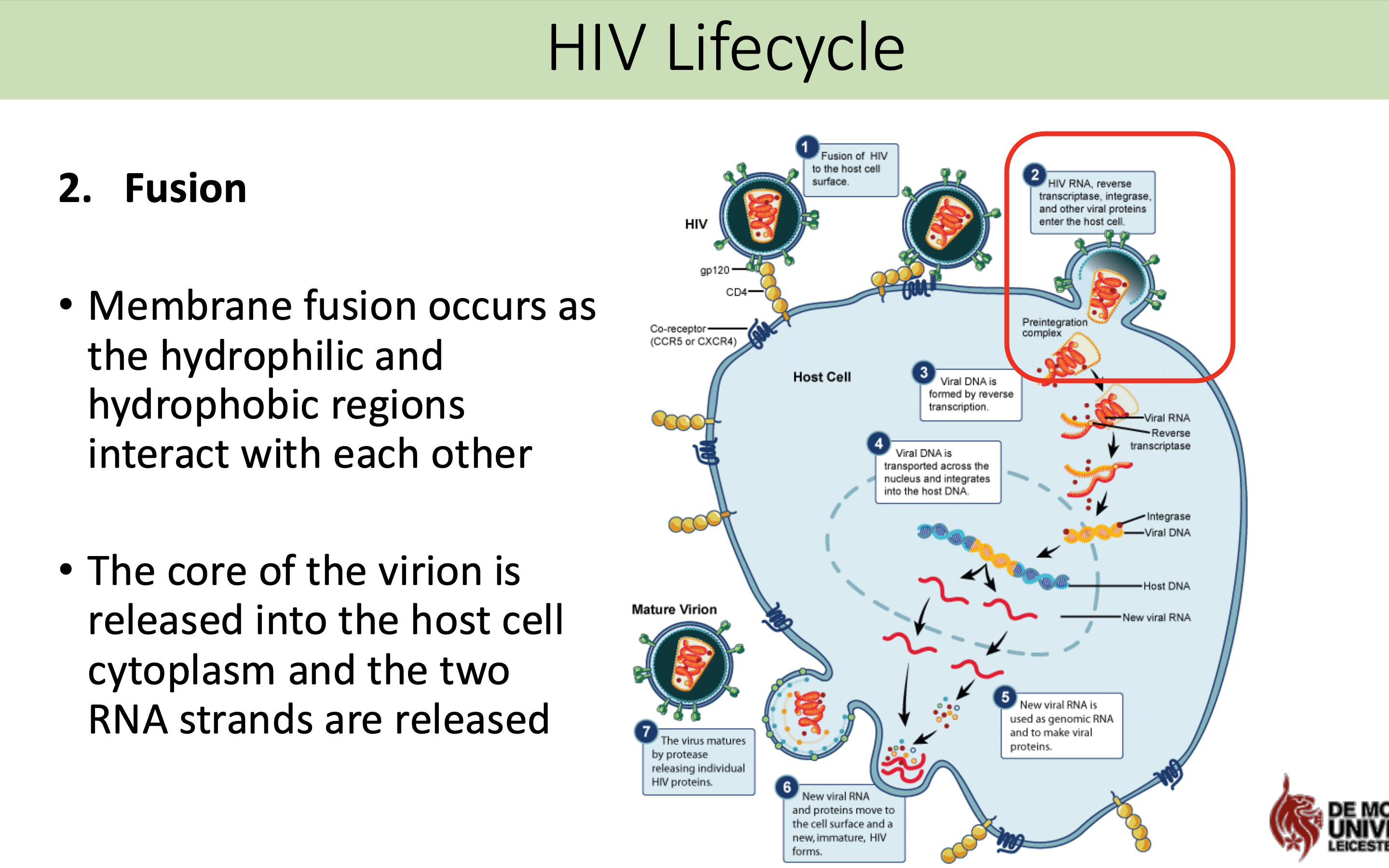
What happens in fusion stage in HIV life cycle
Membrane fusion occurs as the hydrophilic and hydrophobic regions interact with each other
The core of the virion is released into the host cell cytoplasm and the two RNA strands are released
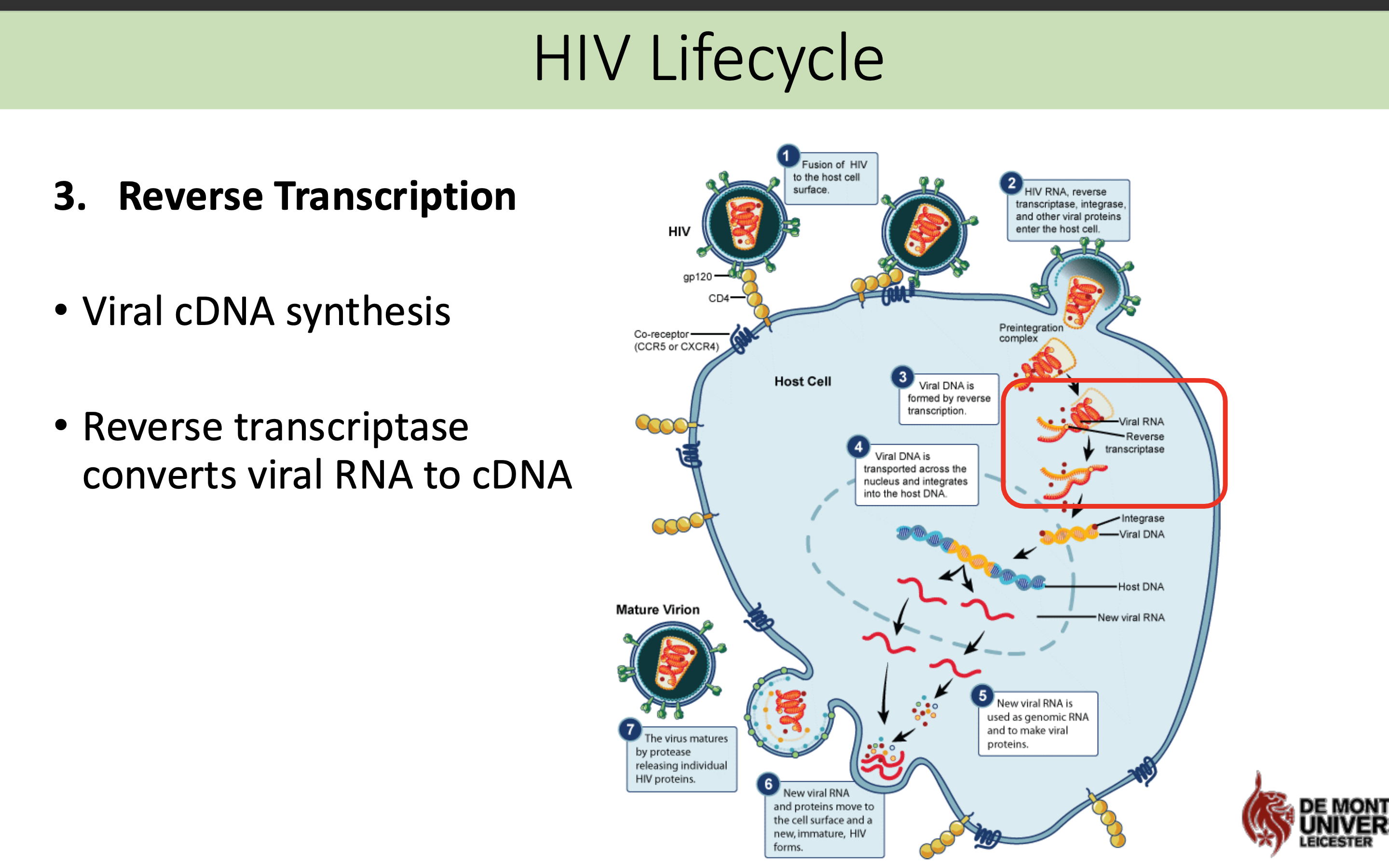
What happens in the reverse transcriptase stage in HIV life cycle
Viral cDNA synthesis
Reverse transcriptase converts viral RNA to cDNA
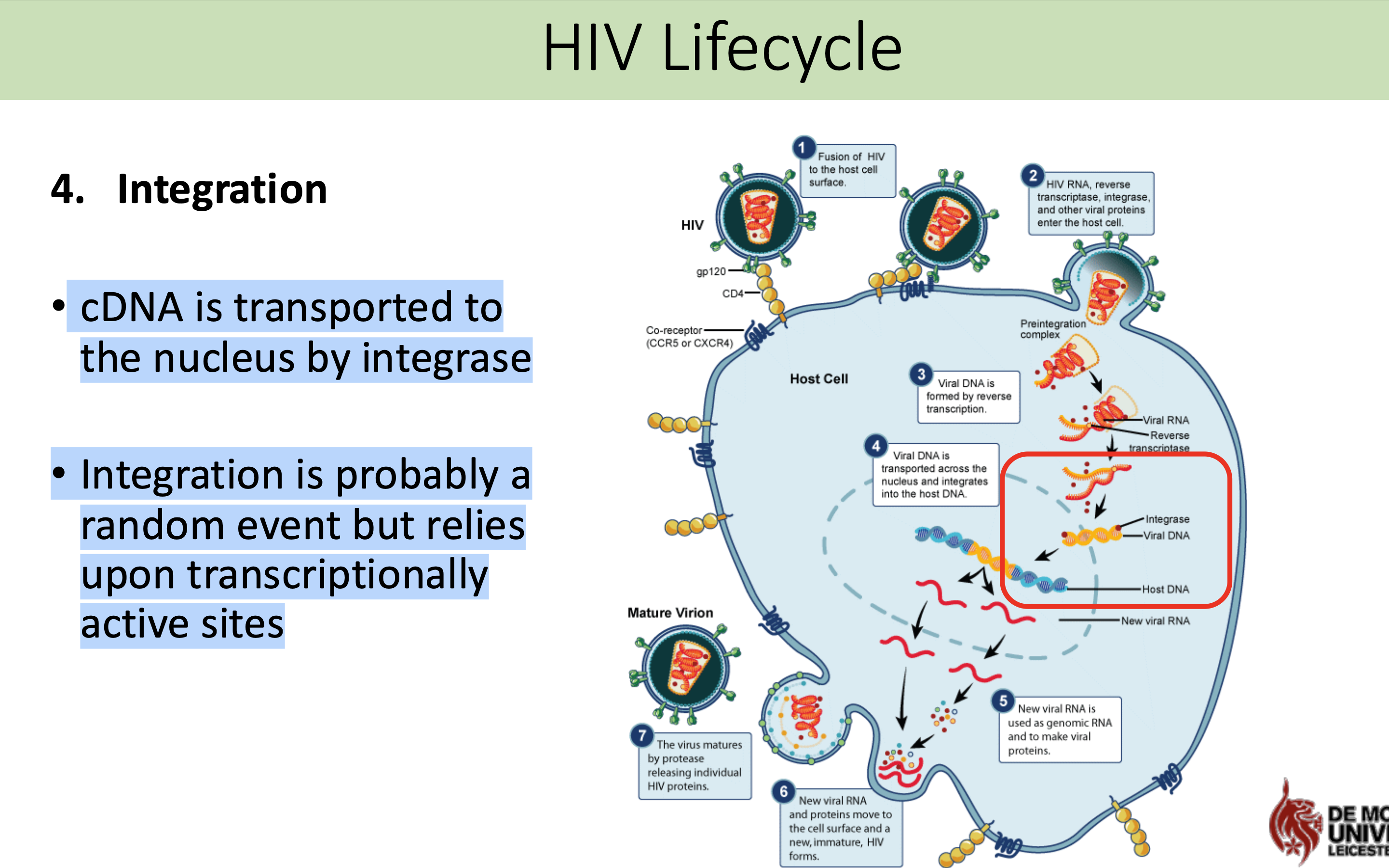
What happens in the integration stage in HIV life cycle
cDNA is transported to the nucleus by integrase
Integration is probably a random event but relies upon transcriptionally active sites
What is viral latency in the HIV life cycle
Viral Latency
• The provirus can silently reside in the host genome or activation?
• The transcription factors NFkB and NF-AT generated during T-cell activation promote transcription of HIV genes
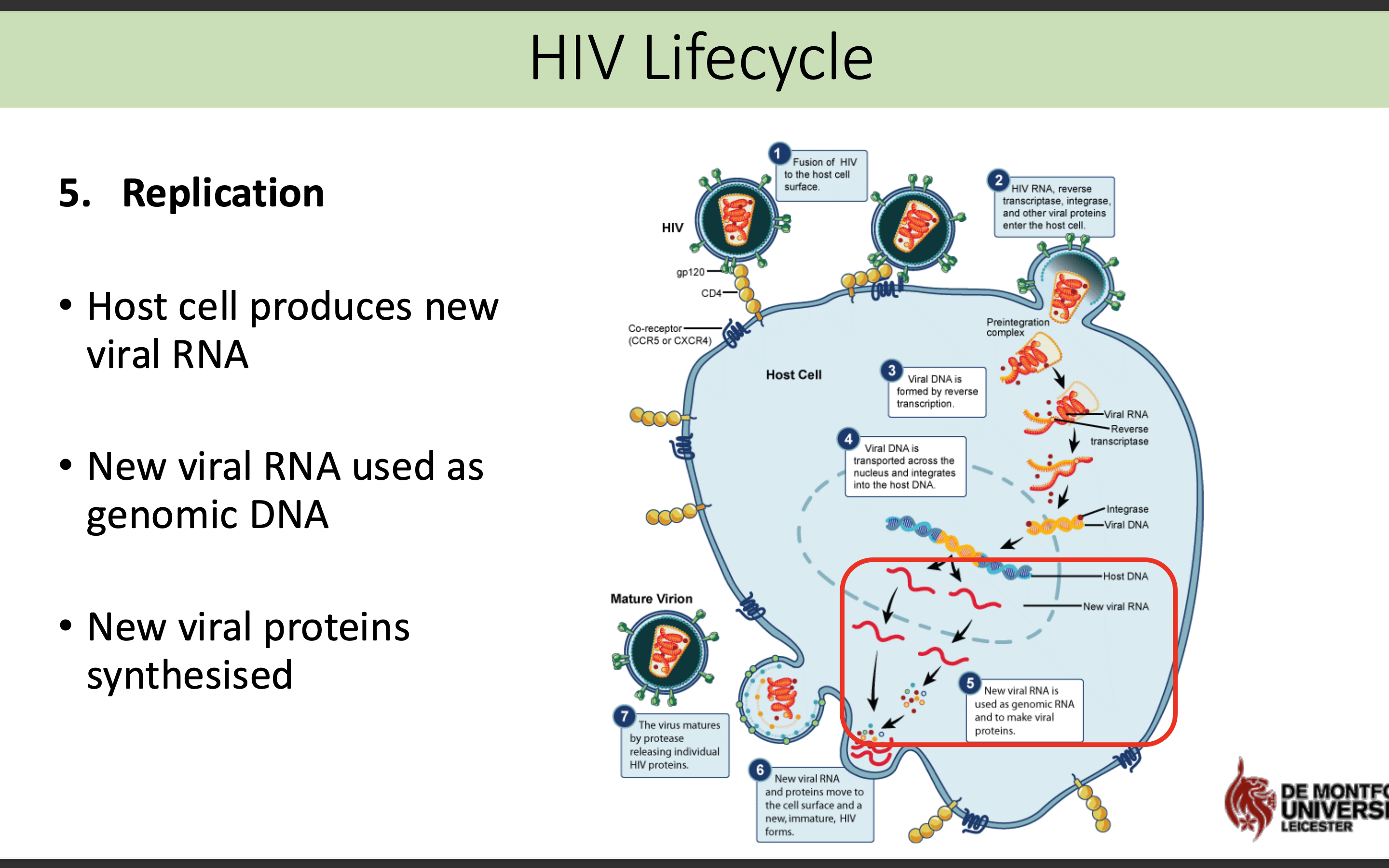
What happens in the replication stage in HIV life cycle
Host cell produces new viral RNA
• New viral RNA used as genomic DNA
• New viral proteins synthesised
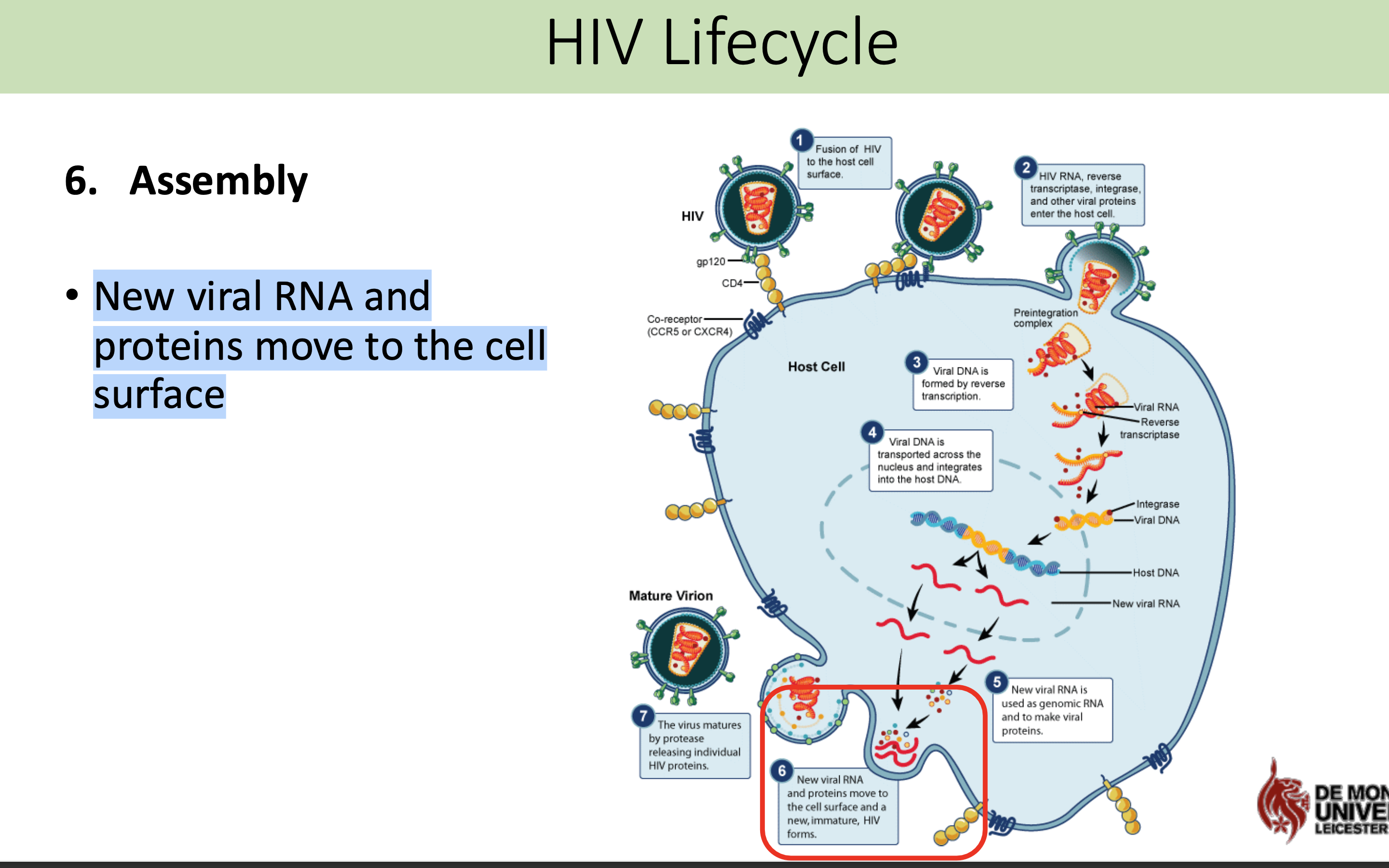
What happens in the assembly stage in HIV life cycle
New viral RNA and proteins move to the cell surface
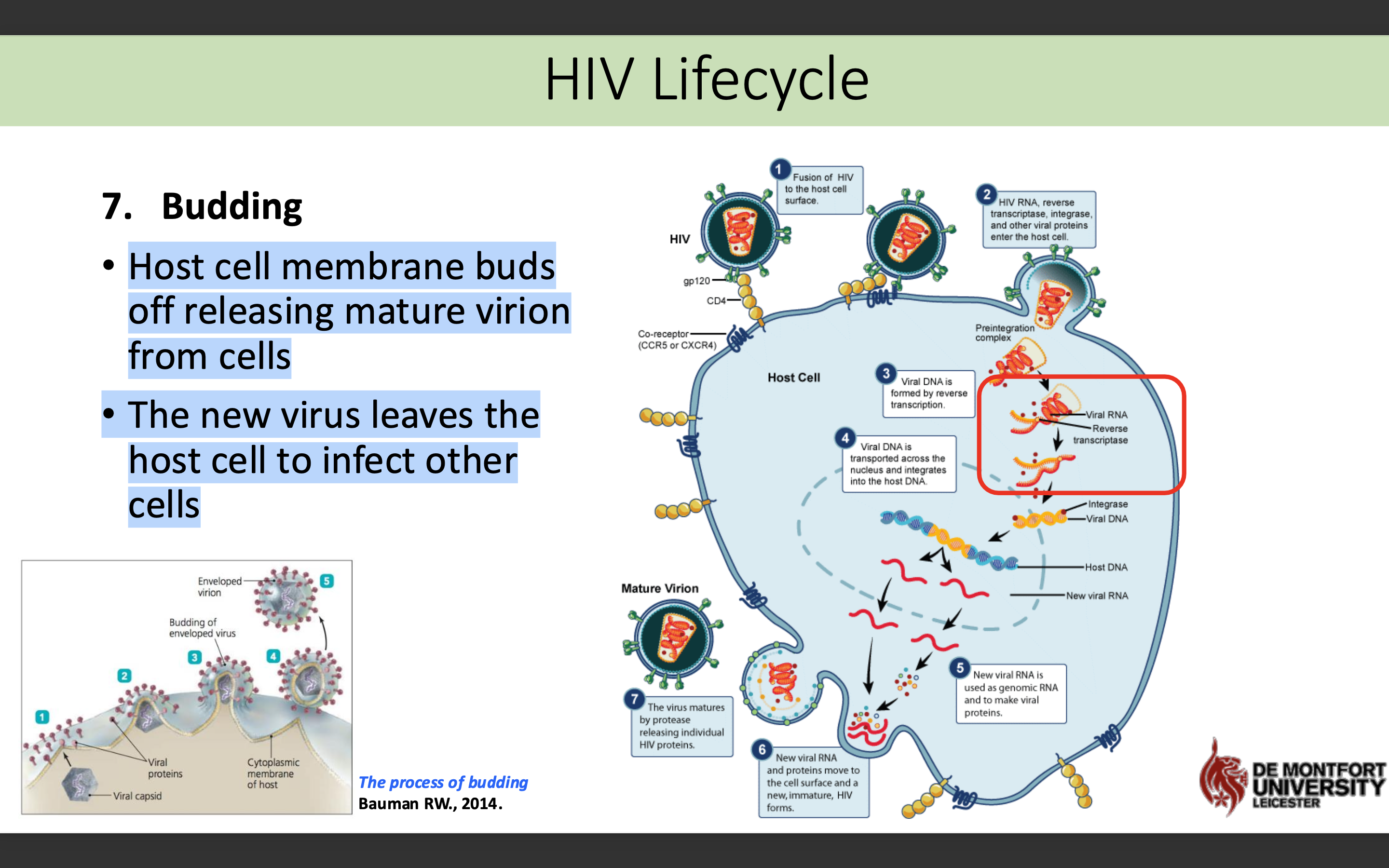
What happens in the budding stage in HIV life cycle
Host cell membrane buds off releasing mature virion from cells
The new virus leaves the host cell to infect other cells
What type of cells does the HIV virus infect
• CD4+ T lymphocytes
• Monocytes
• Macrophages
• Dendritic cells
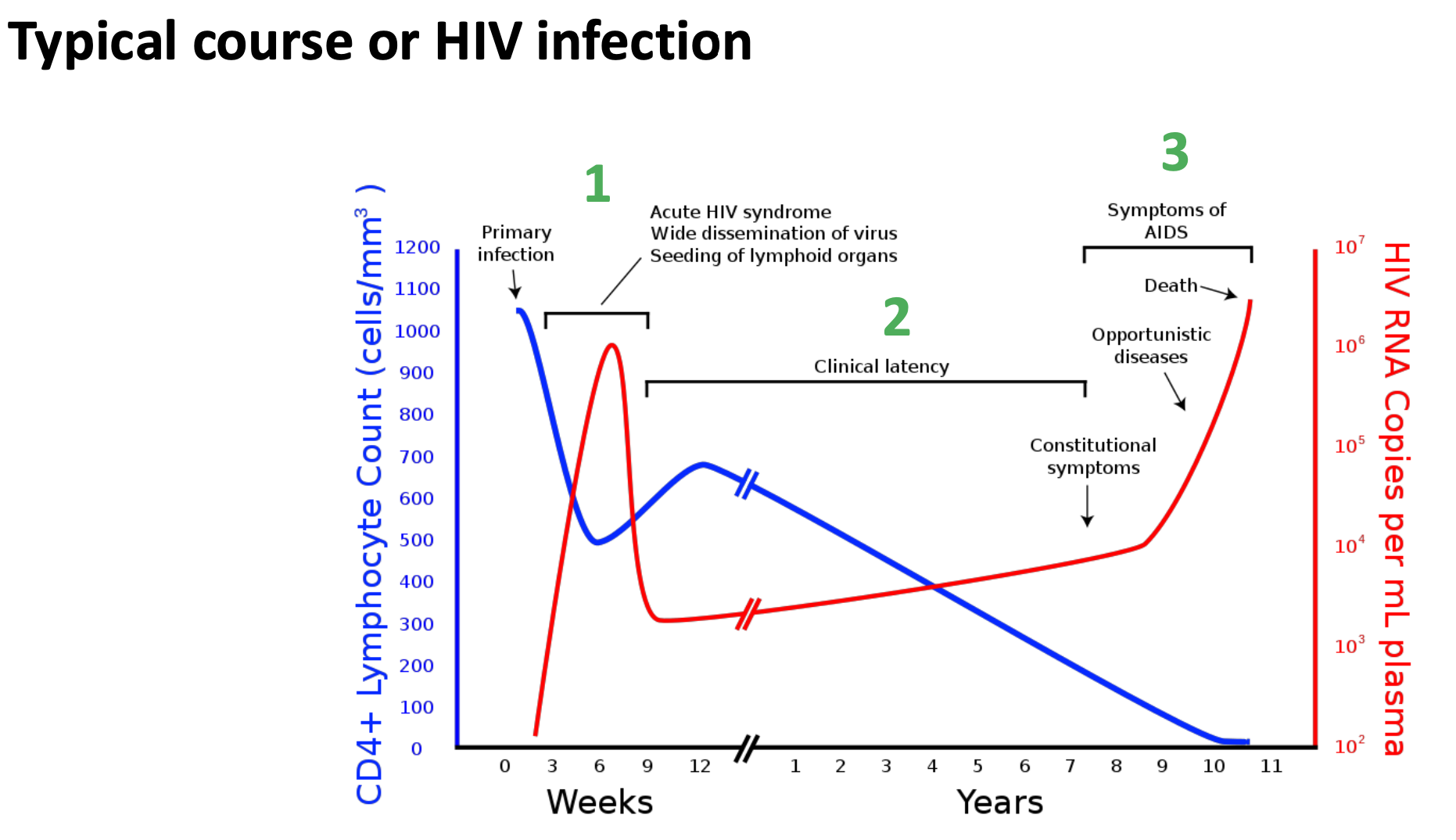
What is the typical course of a HIV infection
The typical course of an HIV infection involves an acute phase with flu-like symptoms, followed by a clinical latency stage where the virus remains inactive, and eventually progresses to AIDS if untreated, characterized by severe immune system damage.
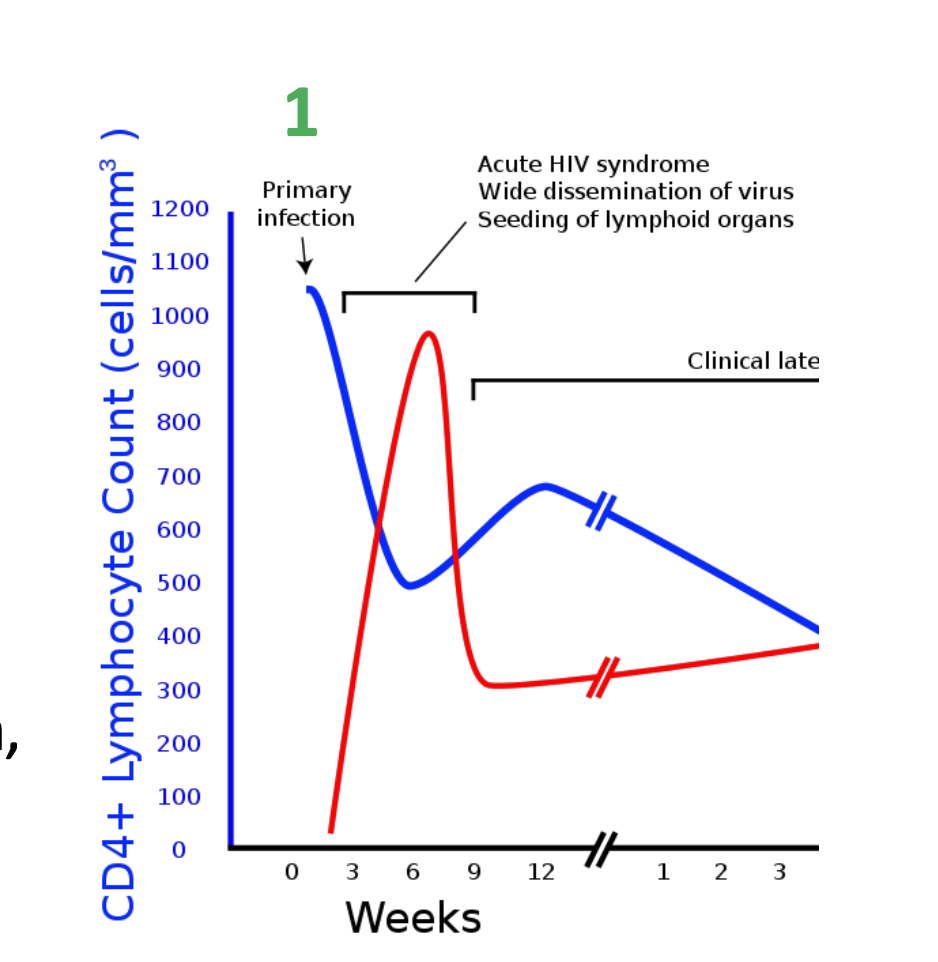
What is stage 1 of the effect of HIV on the immune system
Stage 1 – Primary HIV infection
First few months following infection virus rapidly replicates (CD4+ count reduces)
Asymptomatic infection
Patient does not experience symptoms and may be unaware of infection
Acute retroviral seroconversion syndrome
Acute clinical illness (fatigue, fever, rash, myalgia, pharyngitis, encephalopathy, meningitits)
Persistent generalized lymphadenopathy
Persistent lymph node enlargement
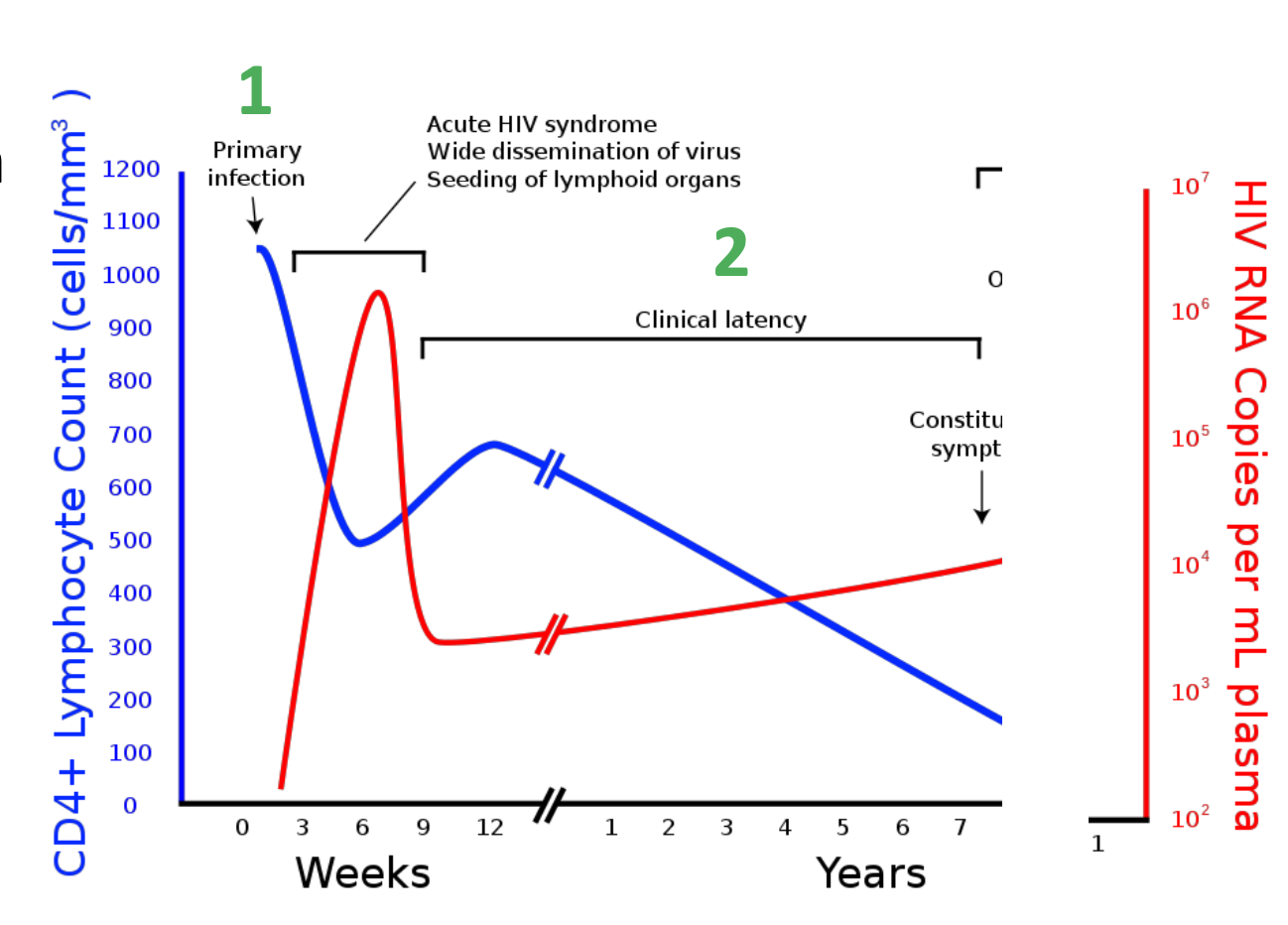
What is stage 2 of the effect of HIV on the immune system
Stage 2 – clinical latency
patient recovers from initial infection
CD4+ count initially recovers
Patient continues to be asymptomatic
Slow and progressive loss of CD4+ cells
HIV viraemia increases
Can last for years
What is stage 3 of the effect of HIV on the immune system
Stage 3 – symptomatic disease
Patients health declines due to decreasing CD4+ count
Opportunistic infections and secondary cancers occur
Early symptomatic disease
Candidiasis
Herpes zoster (shingles)
salmonella
Bacillary angiomatosis (Bartonella)
Also: Stage 3 – late symptomatic disease (AIDS)
– Lymphocytopenia (CD4+ T-cell count <200 cells/mm3)
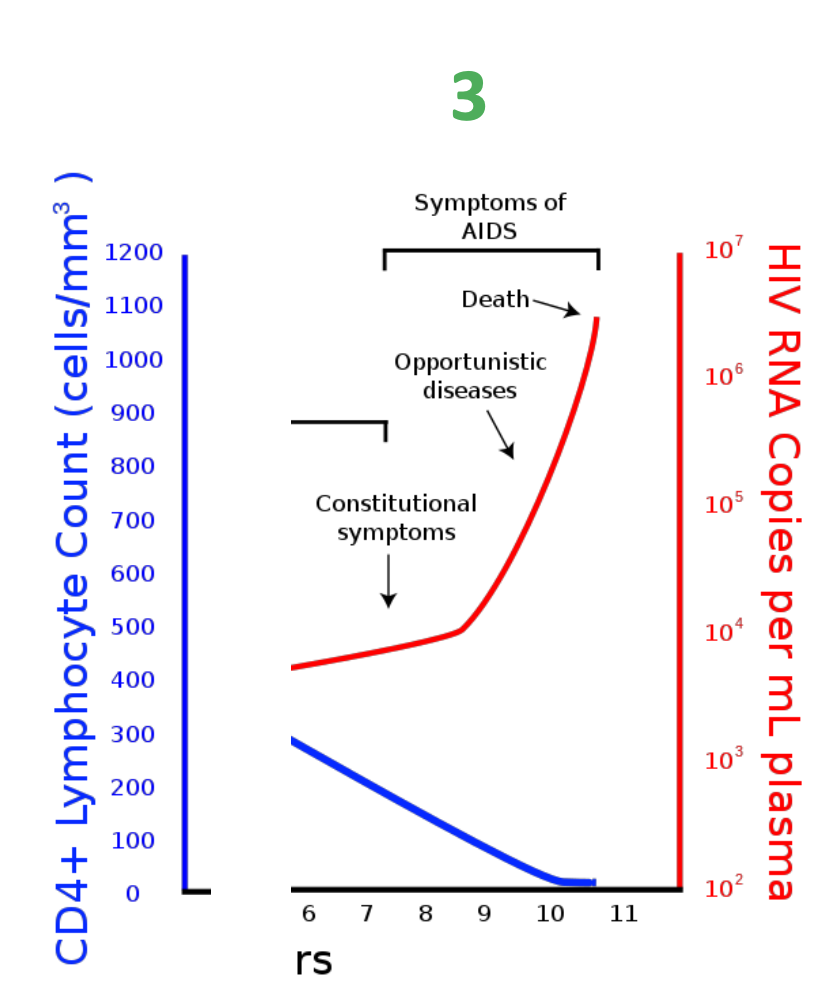
What is AIDS
AIDS (Acquired Immunodeficiency Syndrome) is the late stage of HIV infection characterized by a severely weakened immune system
What is the criteria to meet AIDS
patient who tests positive for HIV and meets at least one of the following criteria:
CD4+ T cells <200 cells/mm3
(normal 600-1000/ μl)
CD4+ T cells <200 cells/mm3 & any of the following diseases:
candidiasis, Pneumocystis jiroveci pneumonia,
HIV wasting syndrome, chronic ulcers
Malignant diseases such as Kaposi’s sarcoma
What are the symptpms of HIV
Sore throat
Fever
Enlarged lymph glands
Rash
Muscle aches
Sweating during the night
Twitching
Fatigue
Mouth ulcers
What does the CDC recommend to test HIV
Enzyme-linked Immunosorbent assay (ELISA)
4th generation = HIV Antigen/Antibody Combination Test
• detects HIV in the blood by identifying either the:
• HIV-1 p24 antigen or
• HIV antibodies.
Immunoassay (Multispot HIV-1/HIV-2 Rapid Test)
• To confirm a positive ELISA result
• To distinguish between HIV-1 and HIV-2
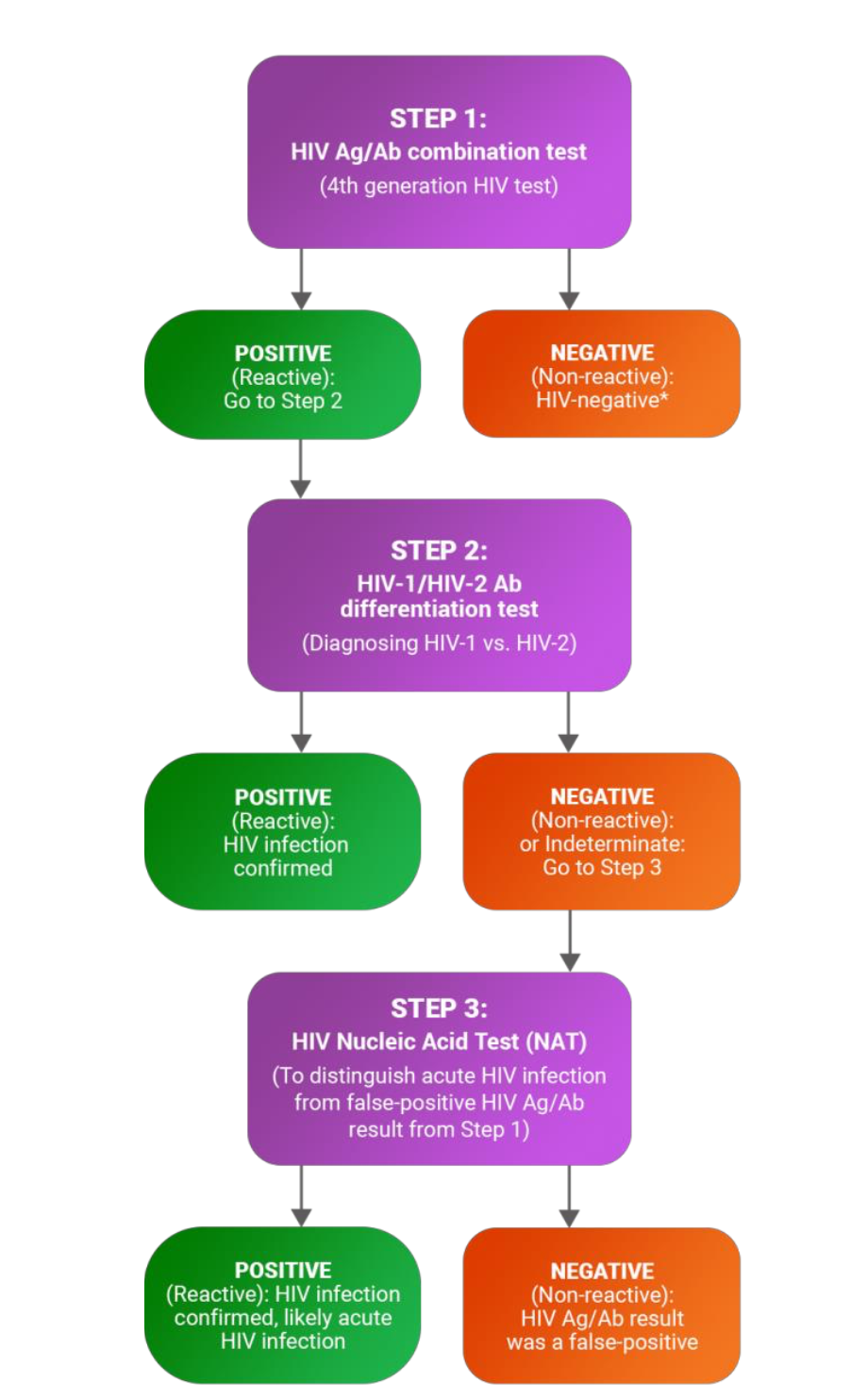
What are some of the additional tests that can be done for HIV
CD4 count (Flow cytometry)
HIV viral load
Explain the additional test of CD4 count
reflects the degree of immunosuppression in people infected with HIV
In a healthy person not infected with HIV, the CD4 count usually >500 cells/mm3
People with CD4 counts <200 cells/mm3 are most at risk of HIV-related opportunistic infections and cancers.
If treatment is started at CD4 counts above 500 cells per microlitre, rather than later, prognosis is improved.
Explain the additional tests of HIV viral load
HIV viral load (NATS)
reflects rates of viral replication
measured using a polymerase chain reaction (PCR) test.
A rising viral load may indicate:
non-adherence to ART,
resistance to one or more antiretroviral drugs,
or an interaction with another medication.
HIV viral load
Viral load ranges from undetectable (less than 20–50 copies of viral genome/mL blood) to over a million copies/mL.
degree of viral replication is linked to the rate of CD4 decline and therefore disease progression
when viral load is suppressed through ART, CD4 counts recover and risk of HIV-related opportunistic infections and cancers declines.
What are the treatments for HIV
Antiretroviral therapy (ART)- Treatment is not a cure - the infection remains
main goal is to reduce a person’s viral load to an undetectable level.
a combination of HIV medicines (a treatment regime) to be taken every day
ART is recommended for all HIV-infected patients
Shown to
reduce disease progression
Decrease comorbid disease
Prevent transmission
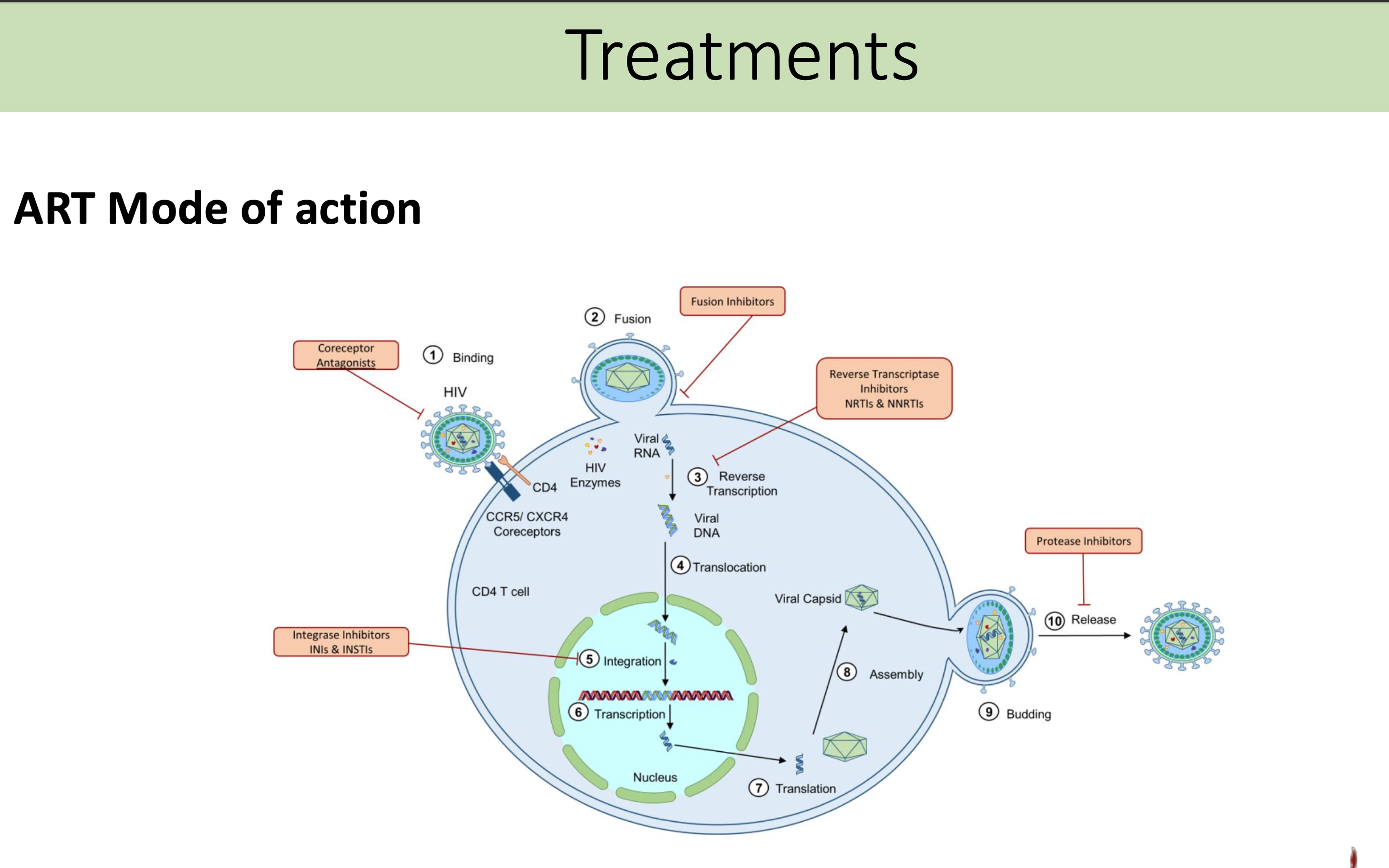
What is the ART mode of action
ART works by inhibiting various stages of the HIV life cycle, including reverse transcription, virus integration, and maturation, thereby reducing the viral load and enhancing the immune response.
What factors can affect ART treatmnet
Poor adherence (not taken correctly)
Pharmacological factors
Host factors (e.g. low CD cell count at start of treatment)
Drug resistance*
High error rate of HIV-1 RT and rapid viral replication contribute to extensive genetic variation – emergence of drug resistance.