A Level Edexcel Physics Paper 2
1/206
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
207 Terms
Electron Gun Model Answer
- Low voltage supply heats filament causes thermionic emission/ electrons have enough energy to leave
- Electrons are repulsed from the cathode (filament) and attracted towards the anode
- This electric field causes electrons to accelerate
- There is a hole in the anode which creates a beam for electrons that pass through
- Electrons are then fired through a vacuum (to stop interfering particles) onto a fluorescent screen
Photo Electric Effect Model Answer
- 1 Electron near the surface of a metal gains 1 photon and gains enough energy to be liberated
- The work function is the energy needed for the electron to escape the surface
- increasing intensity will increase the number of photons
- increasing frequency will increase the energy of the photons
- These electrons released are called photoelectrons
- This effect can be explained through E = hf
PhotoElectric Equation
Ekmax = hf - work fucntion
Ekmax = max kinetic energy of freed electrons
hf = energy of photon
work function = the minimum energy to release an electron
Why does the photoelectric effect equation refer to kinetic energy as maximum kinetic energy?
It will not always be an equal energy transfer:
- Some energy may have been transferred to electrons below the surface
- Therefore for these electrons to liberate to the surface there will be heat transfer through collision
- therefore the electrons leaves the surface will less than max
Energy Frequency equation
E = hf
E = energy
h = plank's constant
f = frequency
Work function equation
Work Function = h x threshold frequency
Work Function
the minimum energy needed to liberate an electron from a metal atom
Threshold Frequency
minimum light frequency necessary to liberate an electron from a given metal
Energy is proportional to frequency
Wave Particle Duality Model Answer
Nuclear Binding Energy
The energy needed to separate a nucleus into individual protons and neutrons
E = mc^2
Binding Energy = mass difference x the speed of light^2
How to calculate nuclear binding energy from a nuclear decay equation?
Th -> Ra + a
Mass of Th nuclear-(Mass of Ra nuclear + mass of alpha particle)
Convert u to kg
Multiply by speed of light^2 E = mc^2
Convert to Mev
How to convert u to kg
kg / 1.6605x10^-27
How to convert kg to u
kg / 1.6605x10^-27
What is 1 u?
1.6605x10^-27kg
Converting u to Mev
u x 931.5
Converting Mev to u
Mev / 931.5
Electron Volt
The energy given to an electron by accelerating it through 1 volt of electric potential difference. 1.6 x 10-19 joules.
Model answer for Fission
- Strong nuclear forces that hold the nucleus together only act a small distance
- When a big nucleus absorbs a neutron it deforms the nucleus
- The electrostatic repulsion between protons becomes greater than the strong nuclear force
- it then splits (random)
Chain Reaction
As a fission reaction splits it produces 3 neutrons which can go on to split 3 other neutrons creating an exponential chain reaction
Model answer for fusion
- two light nuclei join together to make a heavier nucleus
- This gives out more energy per nucleon than than fission as the increase in binding energy is much larger for fusion reactors
Advantages of Fusion over Fission
- Power output is greater
- Raw materials are cheap and available (hydrogen in water)
- No radioactive waster is produced directly
Why Fusion is hard to do?
The need for:
- enormous pressure
- enormous temperatures
We need these to get two nuclei close enough together to fuse
- Containment using magnetic fields uses more energy than the reaction yields
We need this because it is the only way to contain plasma from container walls
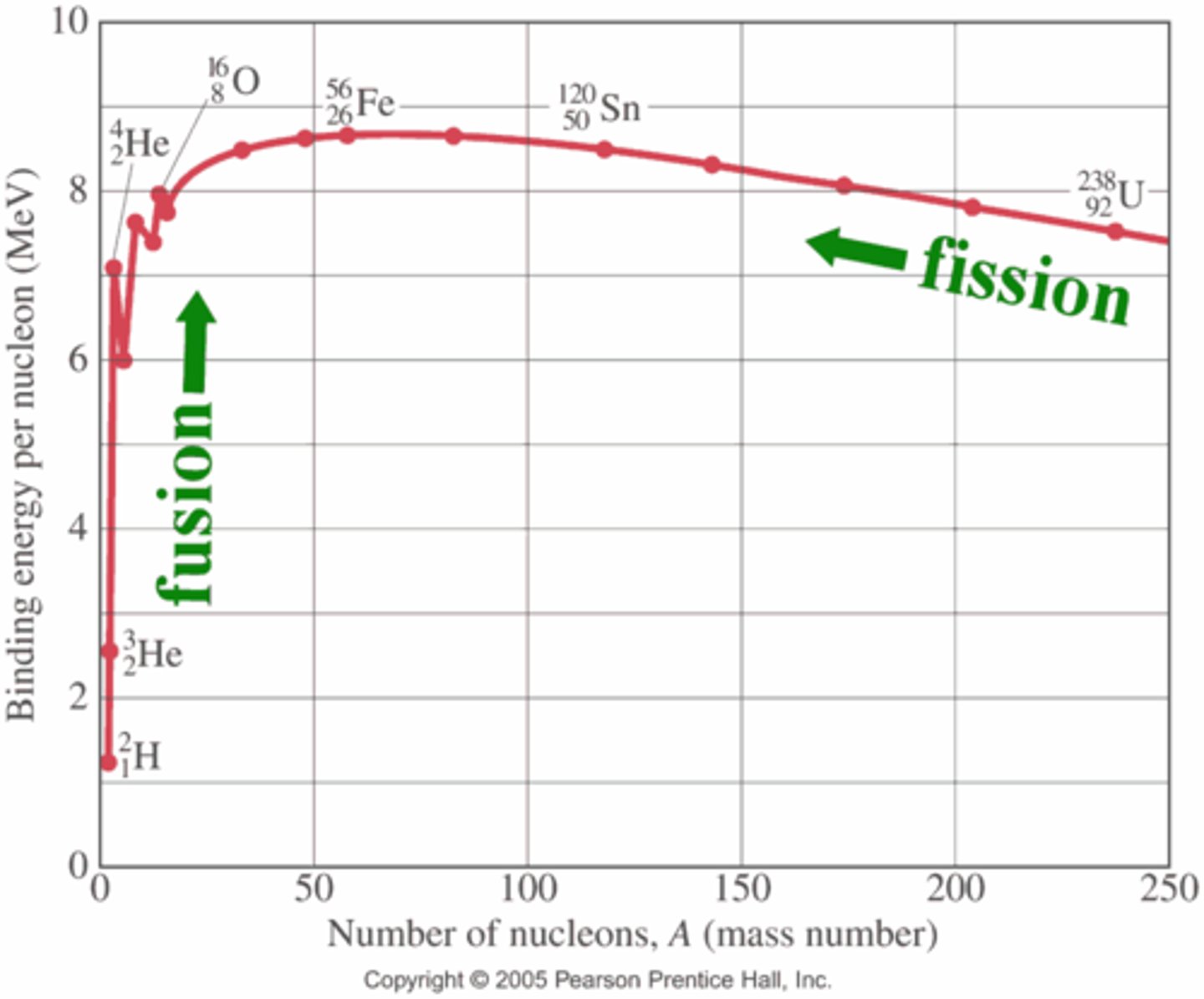
Binding energy per nucleon curve
All reaction through fission or fusion aim to get to iron 56 because it is the most stable element
Absorption Spectra
a spectrum of electromagnetic radiation transmitted through a substance, showing dark lines or bands due to absorption of specific wavelengths.
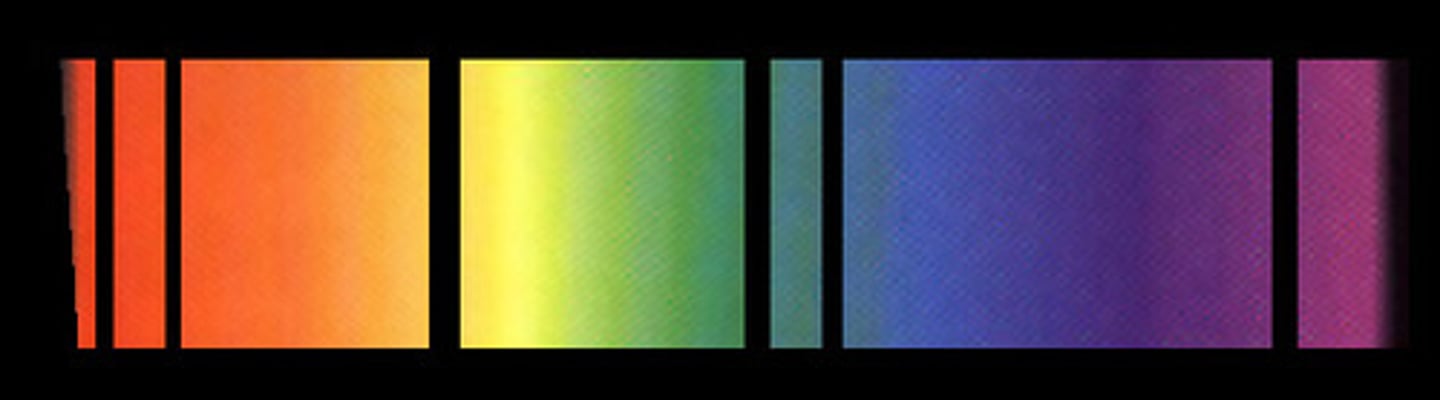
How is an absorption/ emission spectrum produced?
Shining white light through a sample of a gaseous element
The samples of the emission spectrum (the wavelengths missing from the absorption spectrum) are a result of the absorption of energy by electrons corresponding to the energy levels of the element by the gas
Emission Spectrum
a spectrum of the electromagnetic radiation emitted by a source, showing coloured lines due to the emission of the wavelengths
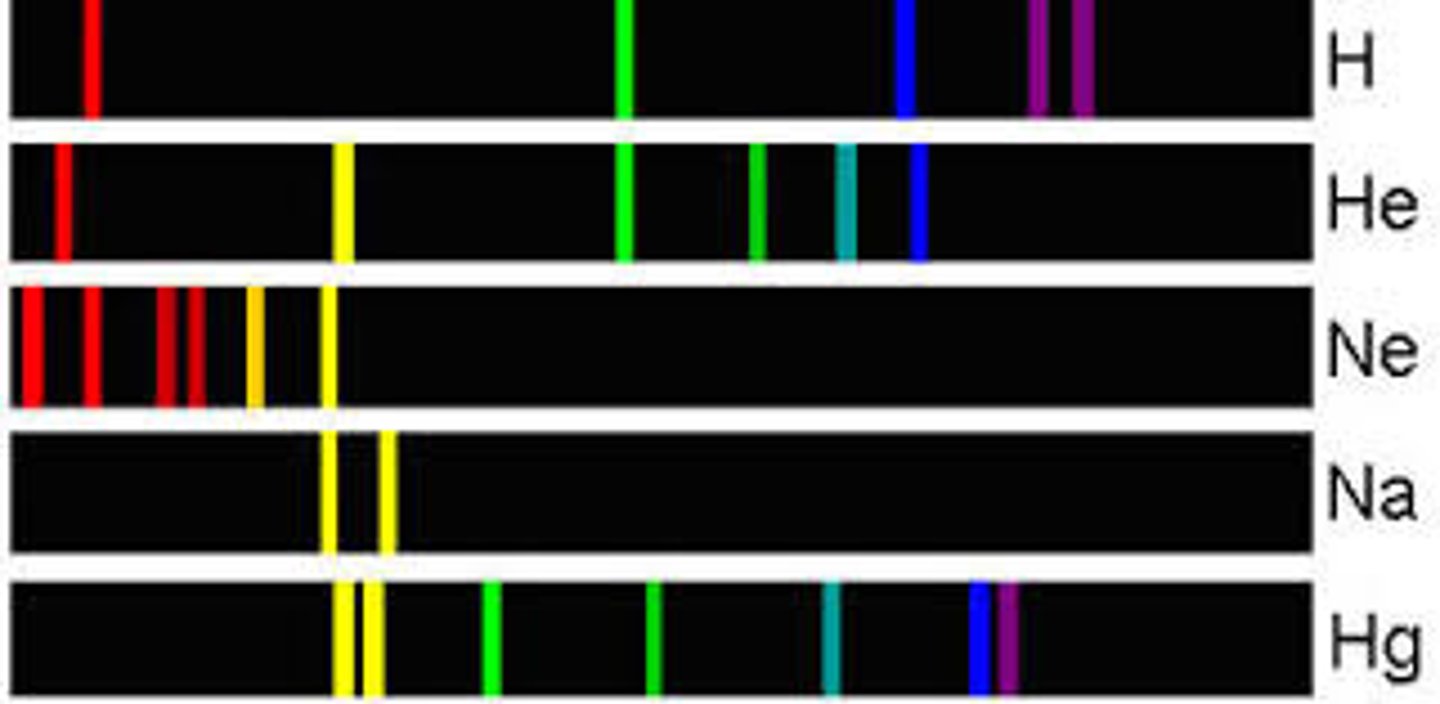
Atomic Line Spectra Model Answer
- All elements and their isotopes have a line spectrum
- The spectra are the wavelengths absorbed or emitted from an element with an electron gains or looses energy
- The energy is lost in the form of photons of certain wavelengths
backround radiation
nuclear radiation that occurs naturally in the environment
Examples of background radiation
- cosmic radiation
- radioactive elements
- radioactive substances
- rocks
- living things
- air
How to account of background radiation?
Measure background count
Subtract this from radioactive element count
Nucleon number
the total number of protons and neutrons in the nucleus of an atom
Mass number
the sum of the number of neutrons and protons in an atomic nucleus
Proton number
the number of protons in the nucleus of an atom
Atomic number
the number of protons in the nucleus of an atom
How to find mass number?
protons + neutrons
The bigger number of element table
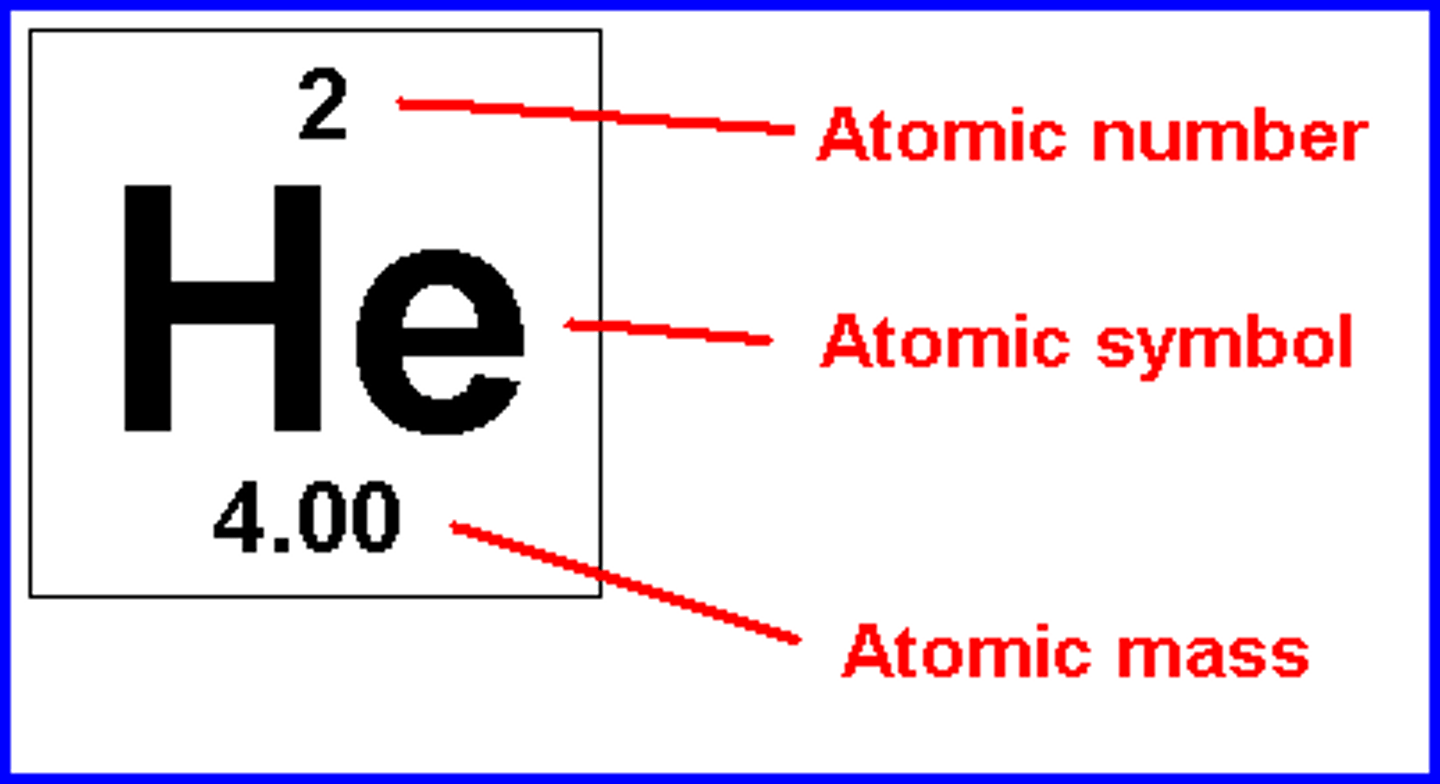
How to find proton/atomic number?
The smaller number on element table
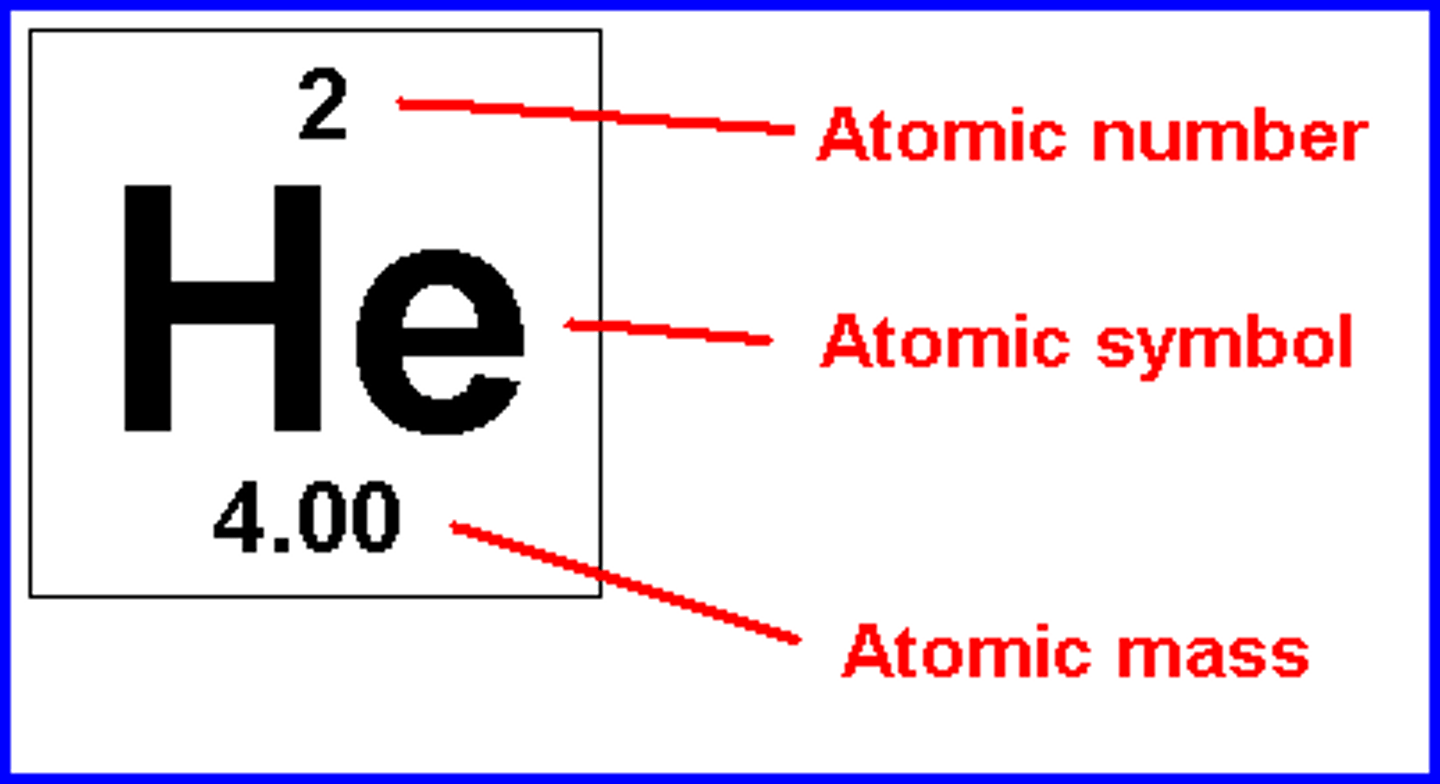
How to find number of electrons?
atomic/ proton number
How to find number of protons?
atomic/ proton number
How to find number of neutrons?
mass number - atomic number
Model Answer for Alpha Particle Scattering Experiment
- Rutherford fired fast moving alpha particles at thin gold foil
- Most alpha particles passed straight through the foil
- Some alpha particles were deflected
- Some alpha particles bounced back
- This meant all of an atoms positive charge was concentrated at the centre of the atom
- The atom was mainly empty space
- Electrons surround the nucleus but at relatively large distances from it
Nature of Alpha Radiation
2 protons and 2 neutrons
A helium nucleas
Nature of Beta Radiation
An electron
Nature of Gamma Radiation
An electromagnetic wave of very short wavelength and high frequency
Mass of radiations
- Alpha = 4u
- Beta = 0.00055 u
- Gamma = 0
Charge of radiations
- Alpha = +2
- Beta = -1
- Gamma = 0
Alpha ionising ability
Very Strong
Beta ionising ability
Medium Strong
Gamma Ionising ability
weak
Symbols of radiaiton
Alpha = a
Beta = e or B
Gamma = y
Speed of alpha radiation
5% of the speed of light
Speed of beta radiation
98% of the speed of light
Speed of gamma radiation
speed of light
Alpha penetration
Stopped by paper, skin or few cm of air
Beta penetration
Stoped by 3mm of aluminium of about 1 m or air
Gamma penetration
reduced significantly by several cm of lead
Electric and magnetic fields effect on radiation
Alpha: yes
Beta: yes
Gamma: no
Kg to Mev/c^2
/ 1.6x10-19
x 10^-6
Mev/c^2 to Kg
x 10^6
x 1.6x10^-19
/(3x10^8)^2
7 SI Units
Mass (kg)
Length (m)
Temp (K)
Illumination (Candela)
Amount of Substance (mole)
Electrical Current (amp)
Time (s)
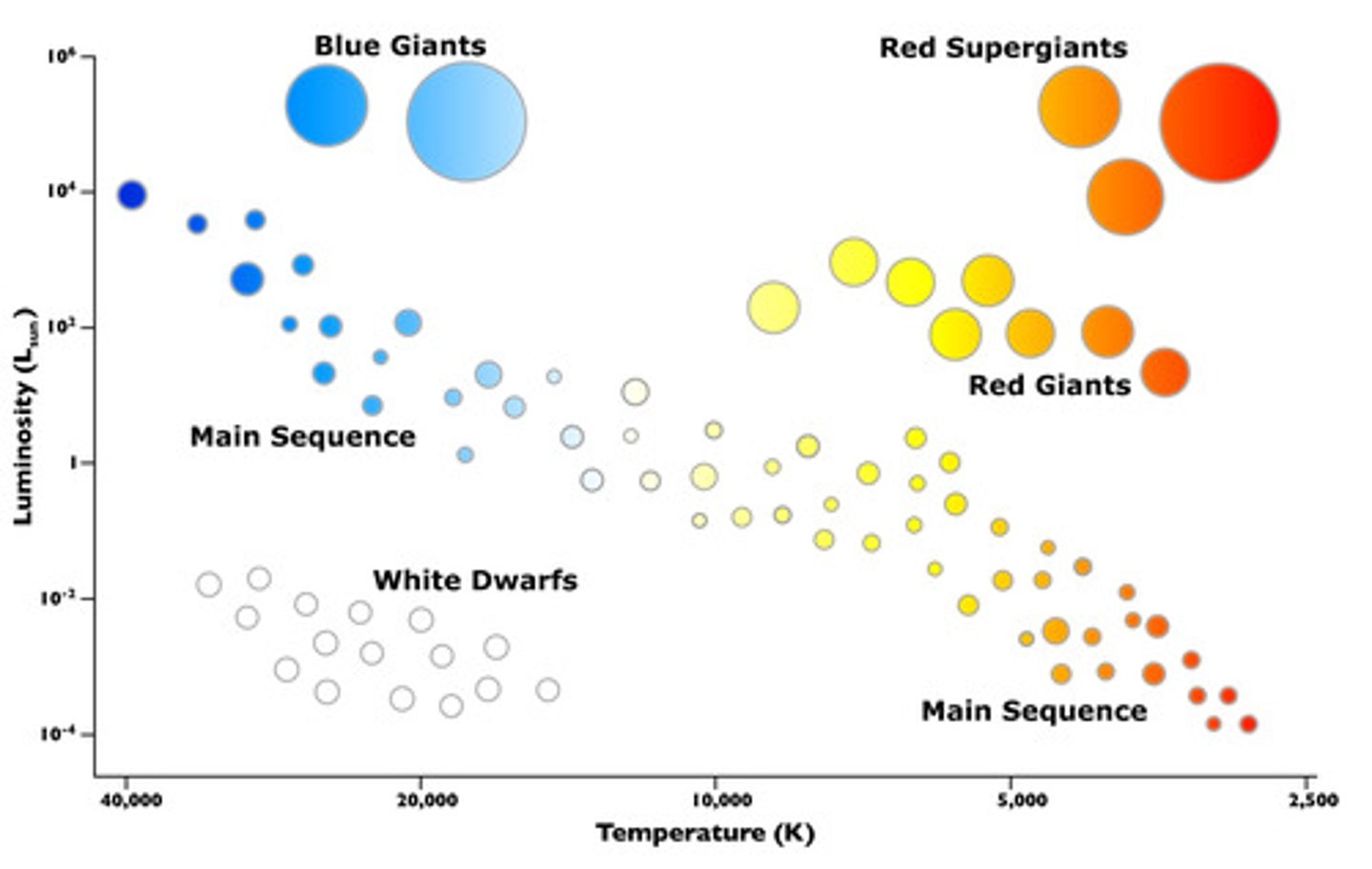
Hertzsprung-Russell diagram
- Temperature Scale goes right to left
- Luminosity is a log scale
- The sun is on main sequence just under red giants
Life Cycle of a small star
nebula, protostar, main sequence star, red giant, white dwarf, black dwarf
Life cycle of a medium star
nebula-protostar, main sequence star, red giant-super nova, neutrons star
Life cycle of large star
nebula, protostar, main sequence star, red giant, super nova, black hole
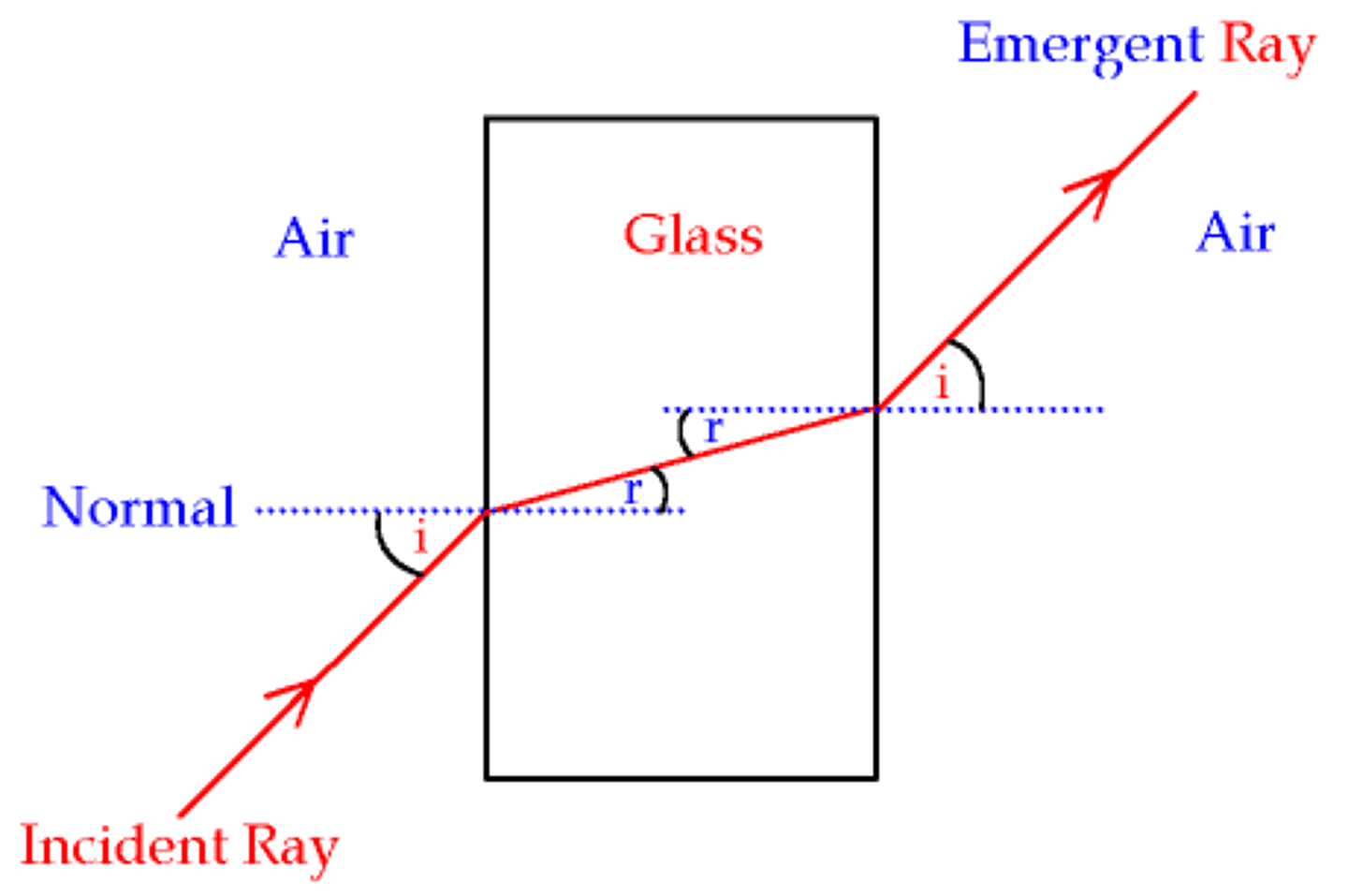
Refraction
The bending of a wave as it passes at an angle from one medium to another
Snell's Law
sini/sinr = refractive index
sin i = angle of incidence
sin r = angle of refraction
Refractive Index Equation
n = c/v
refractive index = speed of light in a vaccum/speed of light in the medium
What is the refractive index of air?
1
Equation for light moving from one medium to another
n1sinangle1 = n2sinangle2
Refractive Index x angle i = Refractive index x angle r
Equation for light moving through several mediums
n1sinangle1 = n2sinangle2
n2sinangle2 = n3sinangle3
n3sinangle3 = n4sinangle4
Ray Diagram for refraction
- Angle of incidence must equal angle of refraction
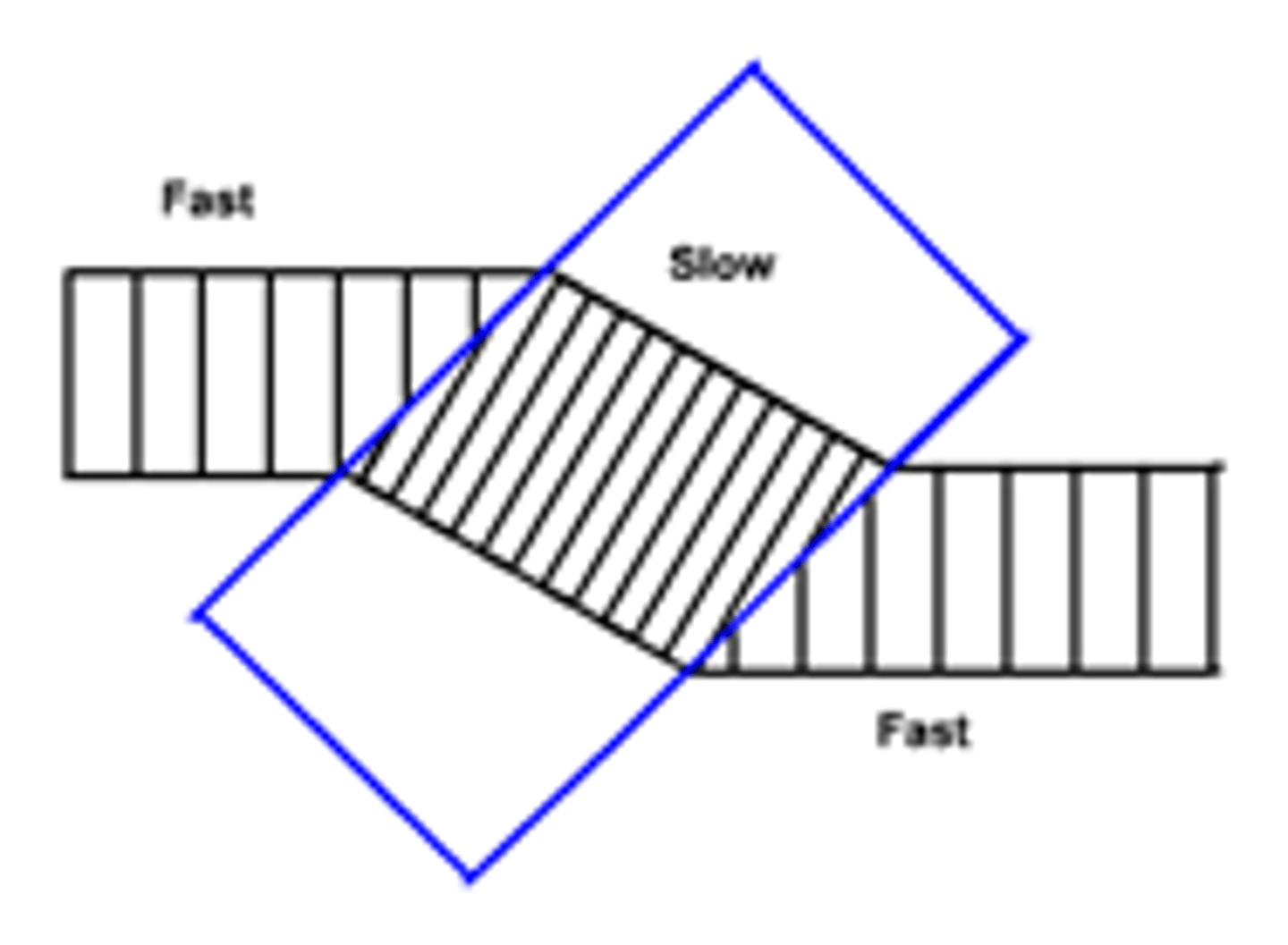
Wavefront Diagram for refraction
- Lines must be equally spaced out
The nature of radioactive decay
- Completely random
- spontaneous as you cannot force a nuclei to decay more than another
- For a particular nuclei there is a probability that it will decay
How to calculate half life?
Half life = ln2/decay constant
Half Life
the time taken for the radioactivity of a specified isotope to fall to half its original value.
Exponential Decay Equations
N = N0 x e^-decay constant x t
A = A0 x e^-decay constant x t
C = C0 x e^-decay constant x t
N = number of nuclei
A = Activity
C = Count rate
Hubbles Graph
- Receding velocity on y
- Distance on x
- Constant = Hubbles constant
- 1 / H is the age of the universe
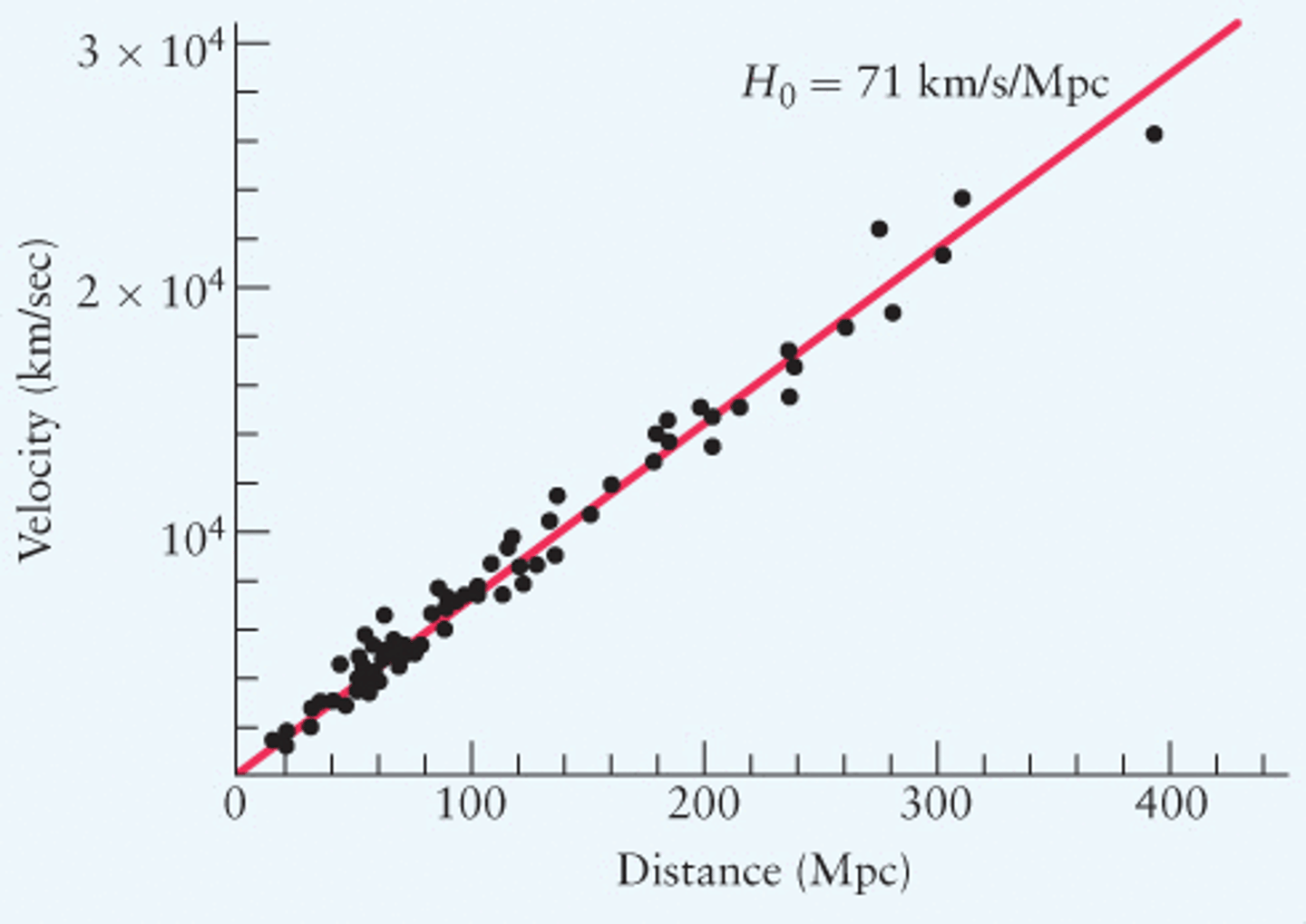
Hubble's Law
v = Hd
v = recessional velocity
H = hubbles constant
d = Distance to that galaxy
How to calculate the age of the universe
t = d/v
v / Hd
t = d/Hd
t = 1/H
Age of the universe
13.7 billion years
Hubbles Constant
70 km/s/Mpc
1 AU in metres
1.5 x10^11m
Distance from earth to sun
1 Light Year in metres
One year =3.15x10^7s
Speed of light = 3x10^8
Distance = speed x time
= 3x10^8 x 3.15x10^7
= 9.45 x10^15m
1 parsec in metres
3.09x10^16m
Distance in pc = 1/parallax angle in seconds of arc
Gravitational Field
the region of space surrounding a body in which another body experiences a force of gravitational attraction.
Gravitational Field Strength
The force acting on each kilogram of mass in the field
g = F/m
Newton's Law of Universal Gravitation
Every object in the universe attracts every other object
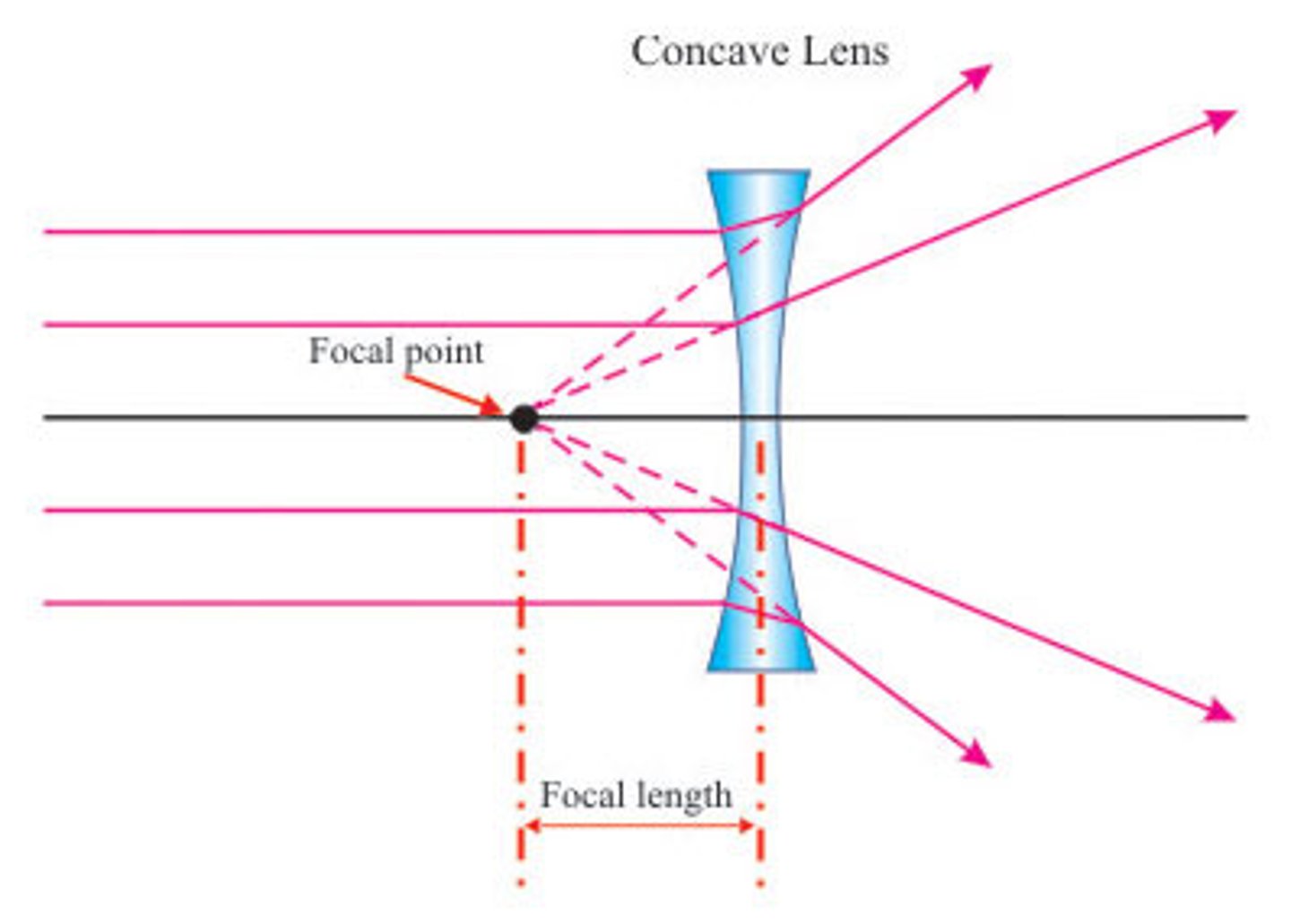
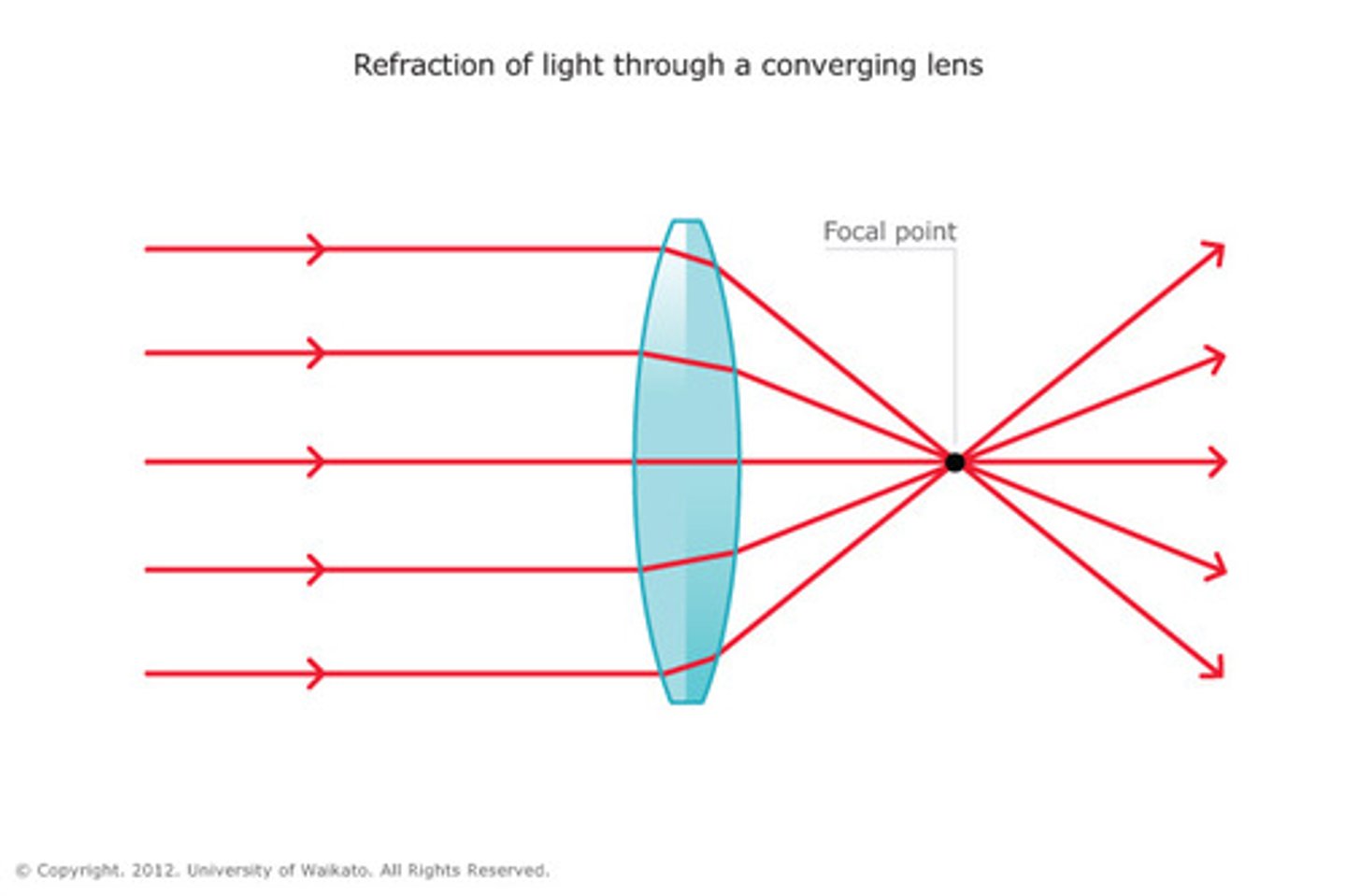
Focal Length
the distance from the optical centre of a lens to the focal point
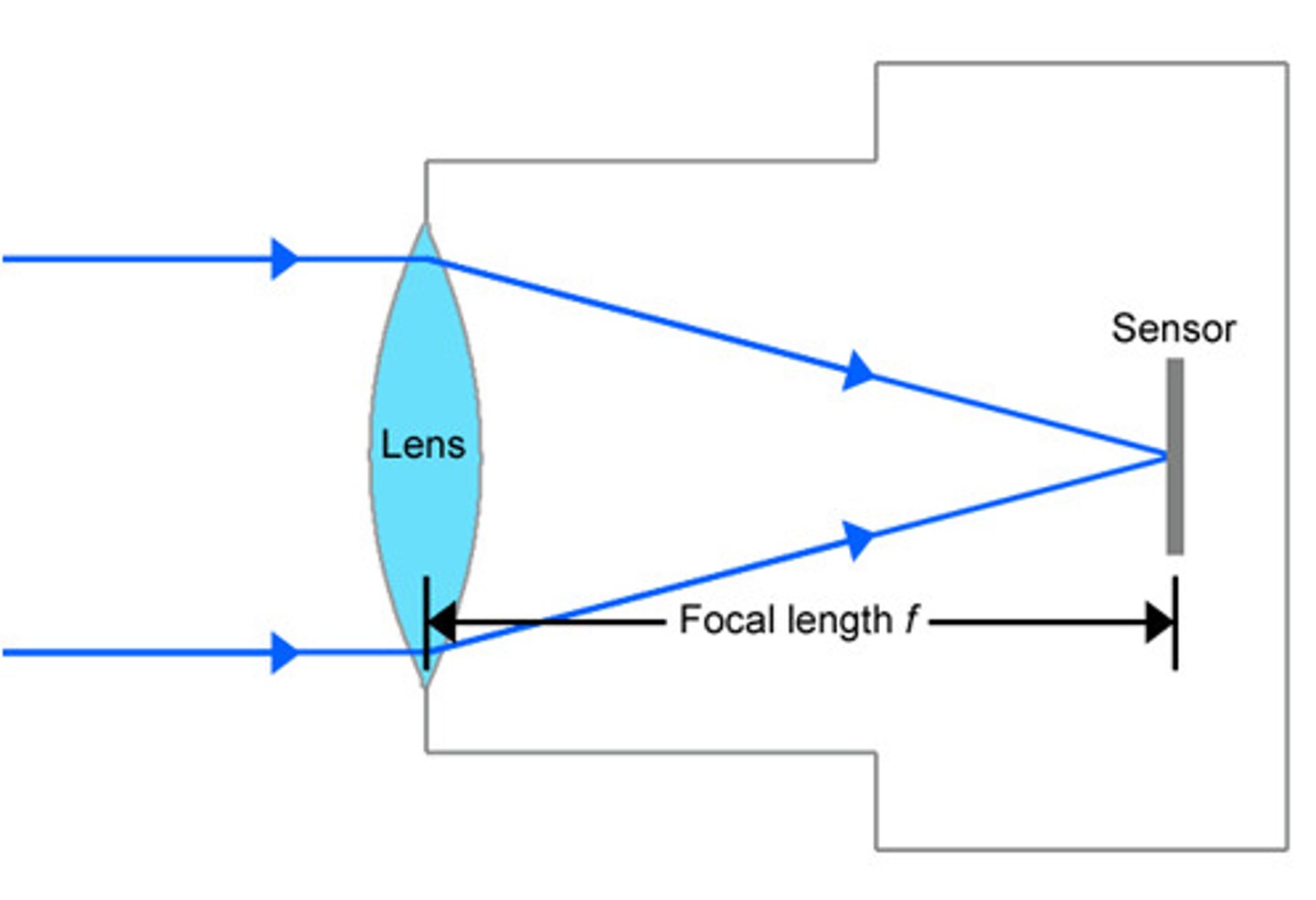
Focal Length Equation
power = 1/f
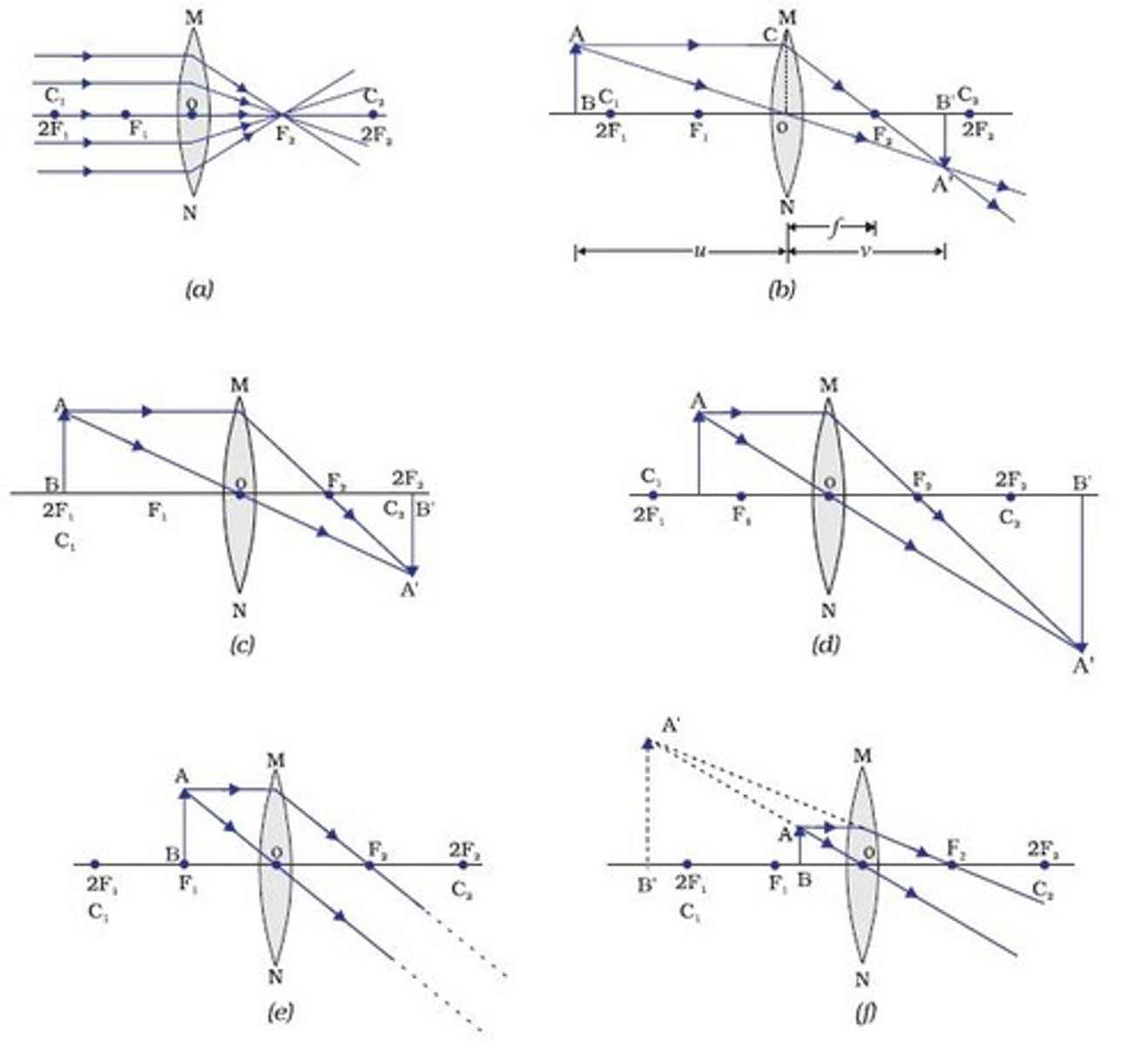
Drawing diagrams for convex lenses where object is after principle focus
1. Draw principle axis and a lens and an arrow to represent the object
2. Mark the principle focuses
3. First ray goes from object to lens then down through the principle focus on the right side
4. second ray goes from top of object through principle focus
5. Draw image where lines meet
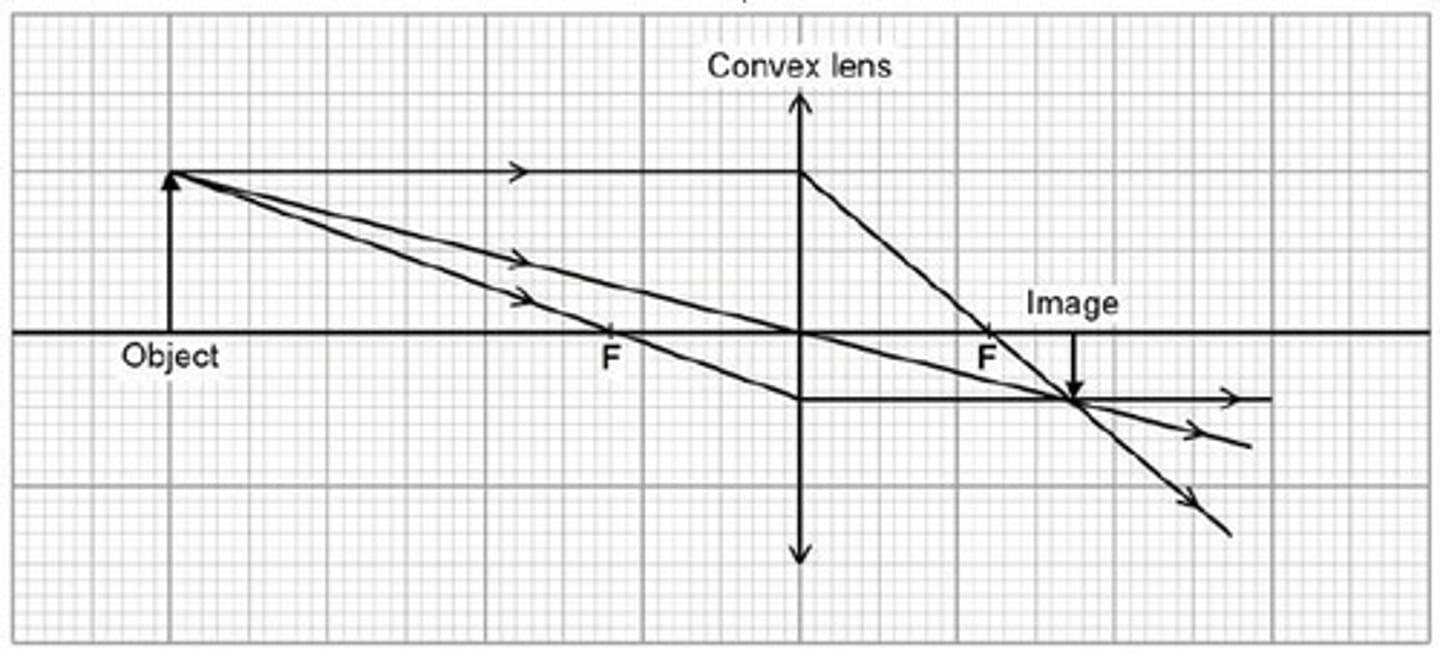
Drawing diagrams for convex lenses where object is before principle focus
1. Draw principle axis and a lens and an arrow to represent the object
2. Mark the principle focuses
3. First ray goes from object to lens then down through the principle focus on the right side
4. second ray goes from top of object through principle focus
5. Trace back rays
6. Draw image where lines meet
Drawing Lens diagram for a concave lens
1. Draw principle axis and a lens and an arrow to represent the object
2. Mark the principle focuses
3. first ray goes from top of object to lens and then goes upward in line with focus
4. Second ray goes from top of object through optical centre
5. Where rays cross is where image forms
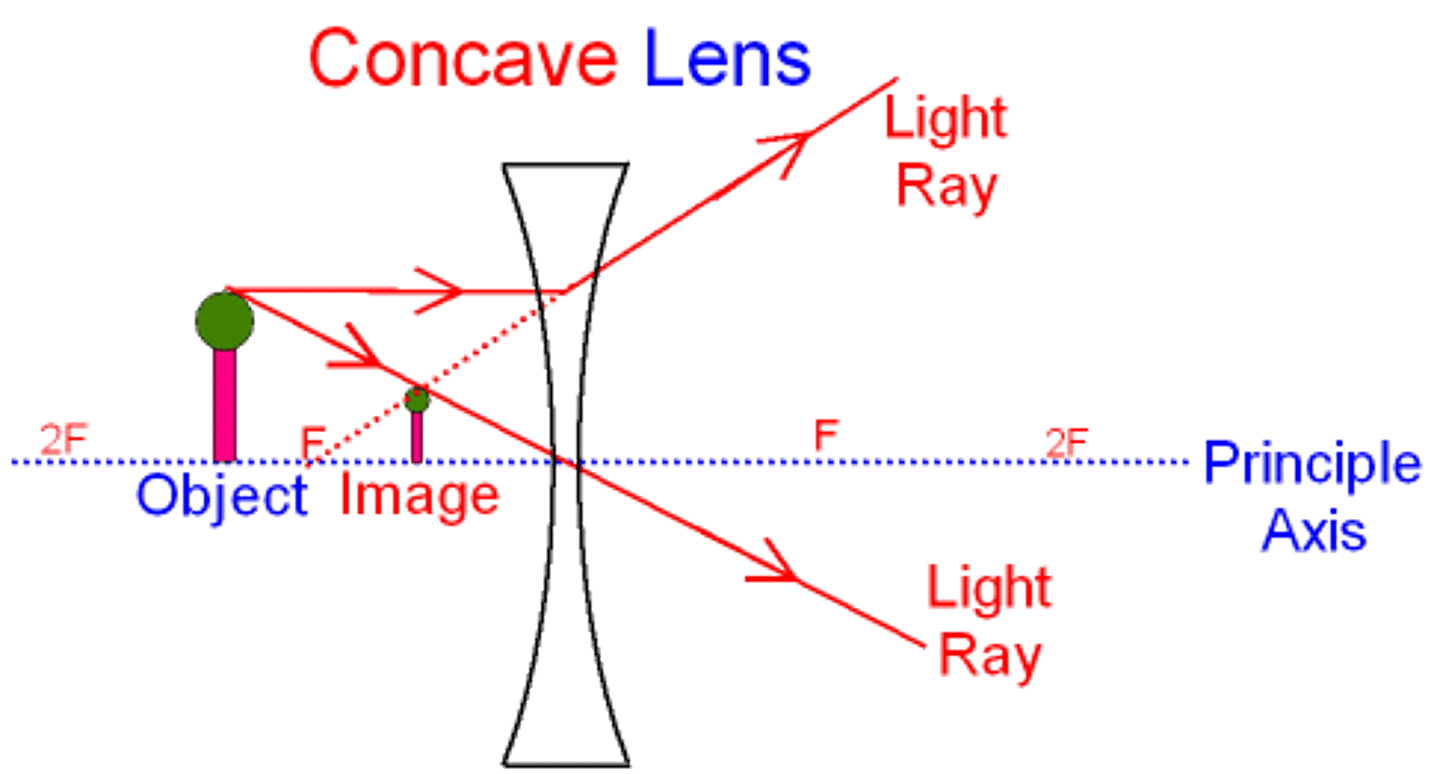
Concave Lens
Diverging
Spreads light
Correct short sightedness
Convex
Converging
Focuses light
Correct far sightedness
Frequency
Number of oscillation in one second
Frequency Equation
f=1/T
Time Period
The time is takes an oscillation to repeat
Time Period Equation
T = 1/f
Wavelength
Horizontal distance between the crests or between the troughs of two adjacent waves
Power in lenses
Power = 1 / focal length
For multiple lenses P = P1 + P2 + P3...
Measured in dioptres D
A more powerful lens the focal length is shorter
Real Image
An image formed on the right side of a lens and can be projected onto a screen upside down