Hybridization, SIgma, and Pi bonds
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
Hybridization
the mixing of several atomic orbitals to form the same total number of equivalent hybrid orbitals
How are sigma bonds formed?
head on overlap of atomic orbitals
How are pi bonds formed?
By parallel overlap of unhybridized p-orbitals
What is a sigma bond?
Overlapping S orbitals
What is a pi bond?
Overlapping P orbitals
What are the exceptions to the octet rule?
hydrogen and helium, (any molecule with and uneven number or valence electrons)
atomic orbitals
the regions around the nucleus within which the electrons have the highest probability of being found
molecular orbitals
an orbital that applies to the entire molecule
bonding orbitals
a molecular orbital that can be occupied by two electrons of a covalent bond
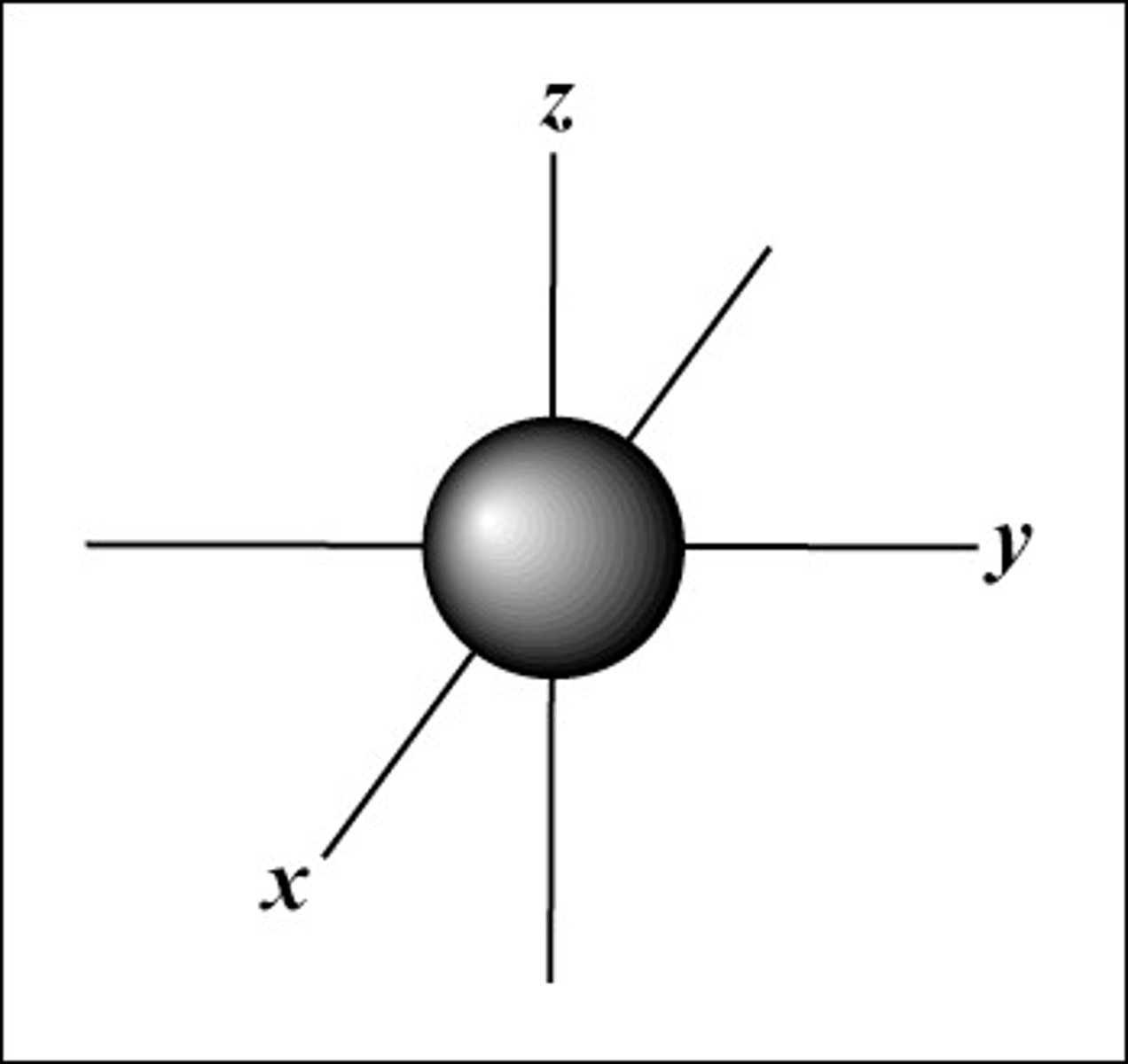
What does the s orbital look like?
sphere
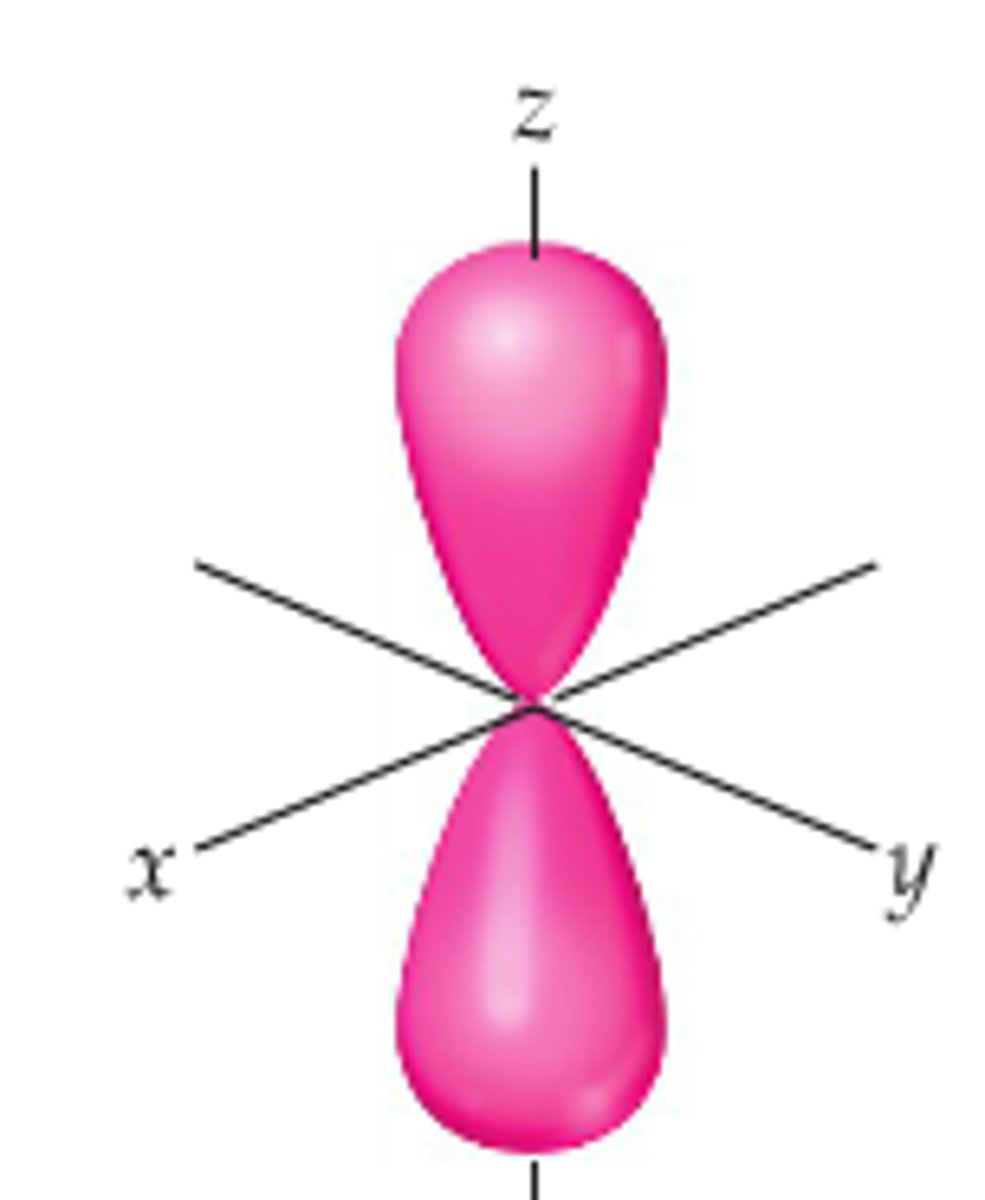
What does a p orbital look like?
dumbbell
Do Pi bonds play in hybridization?
no
Which is stronger a sigma or pi bond?
sigma bond
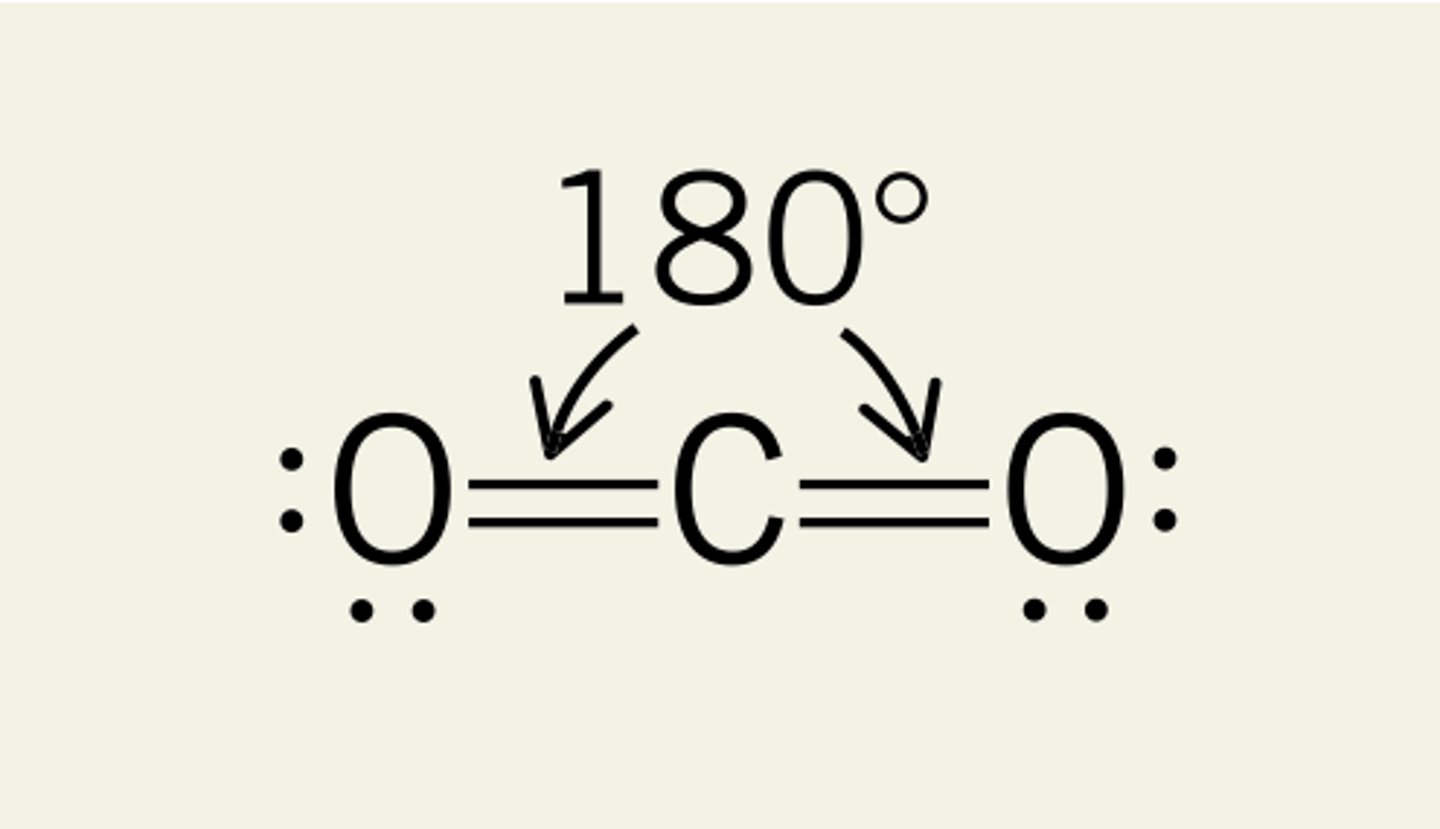
what shape is this?
linear =180
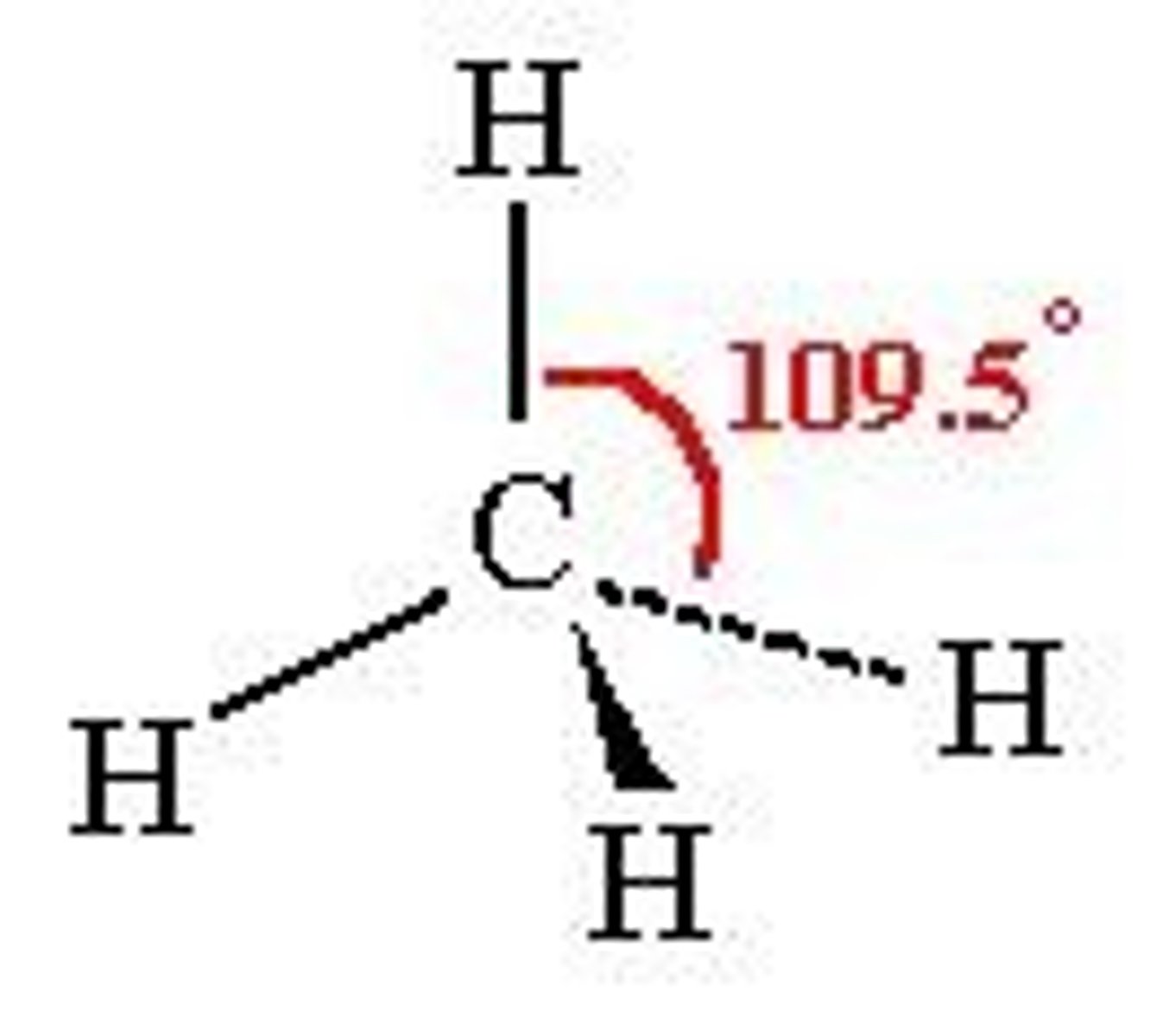
what shape is this?
tetrahedral =109.5
what shape is this?
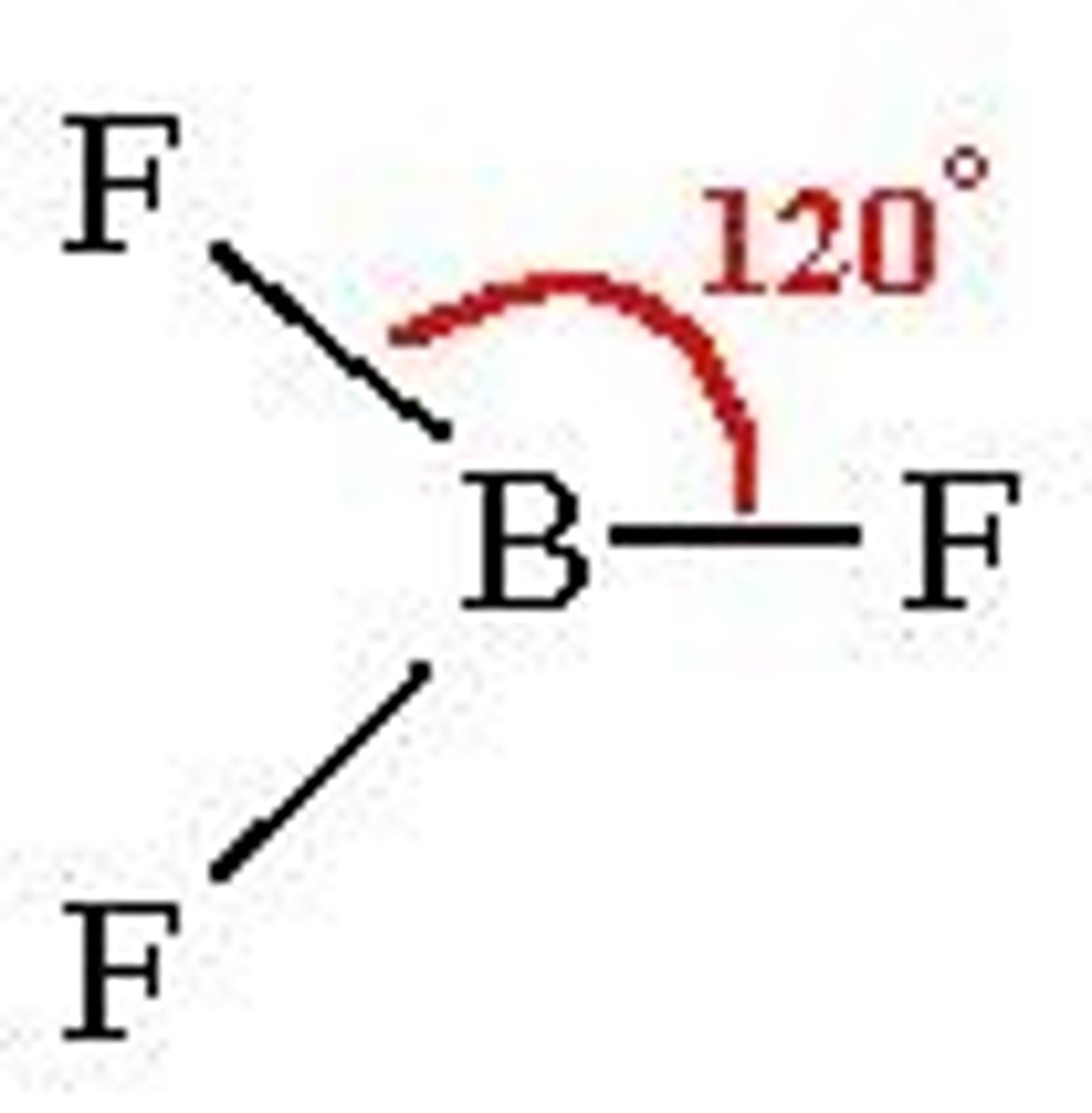
trigonal planar =120
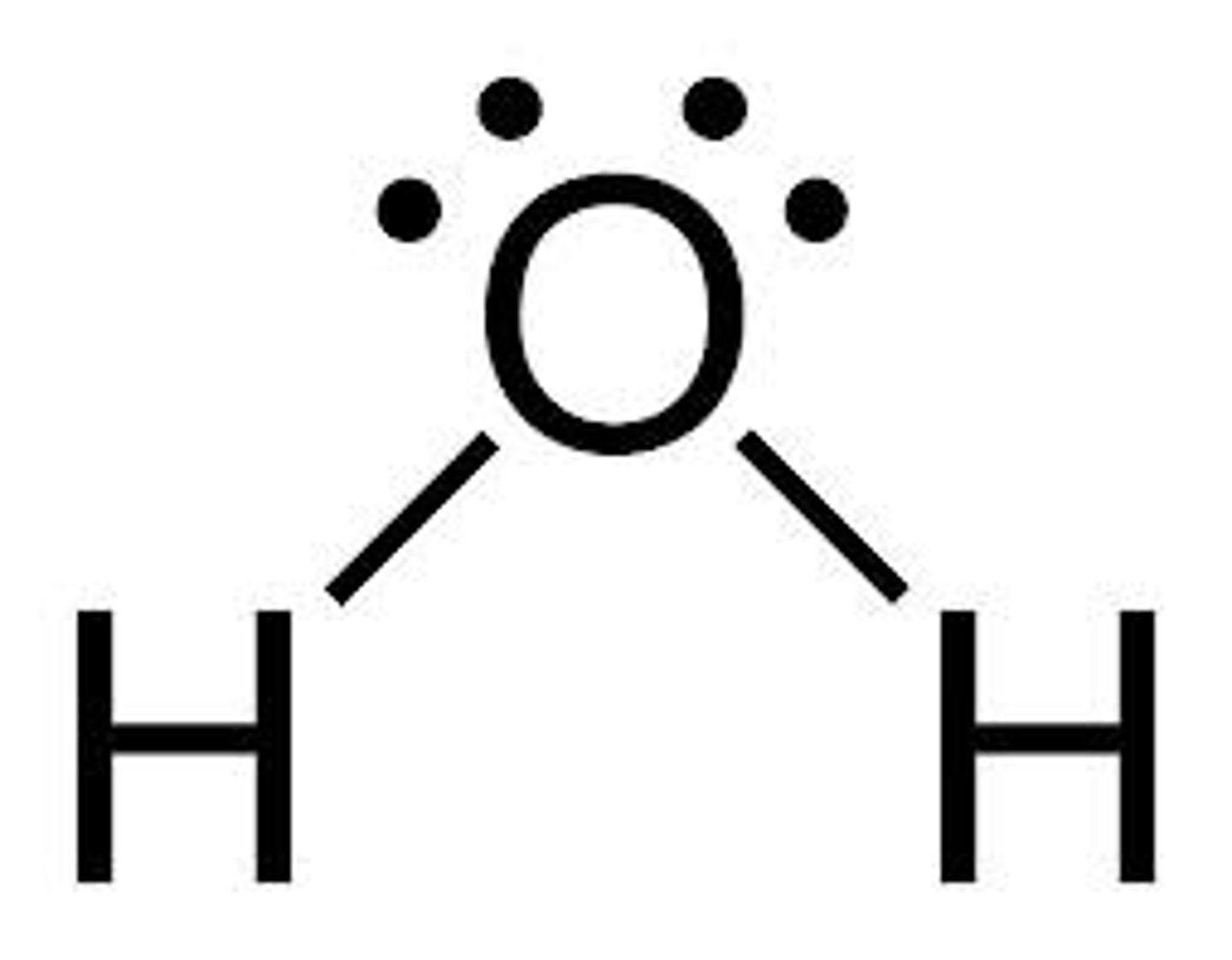
what shape is this?
bent =104.5
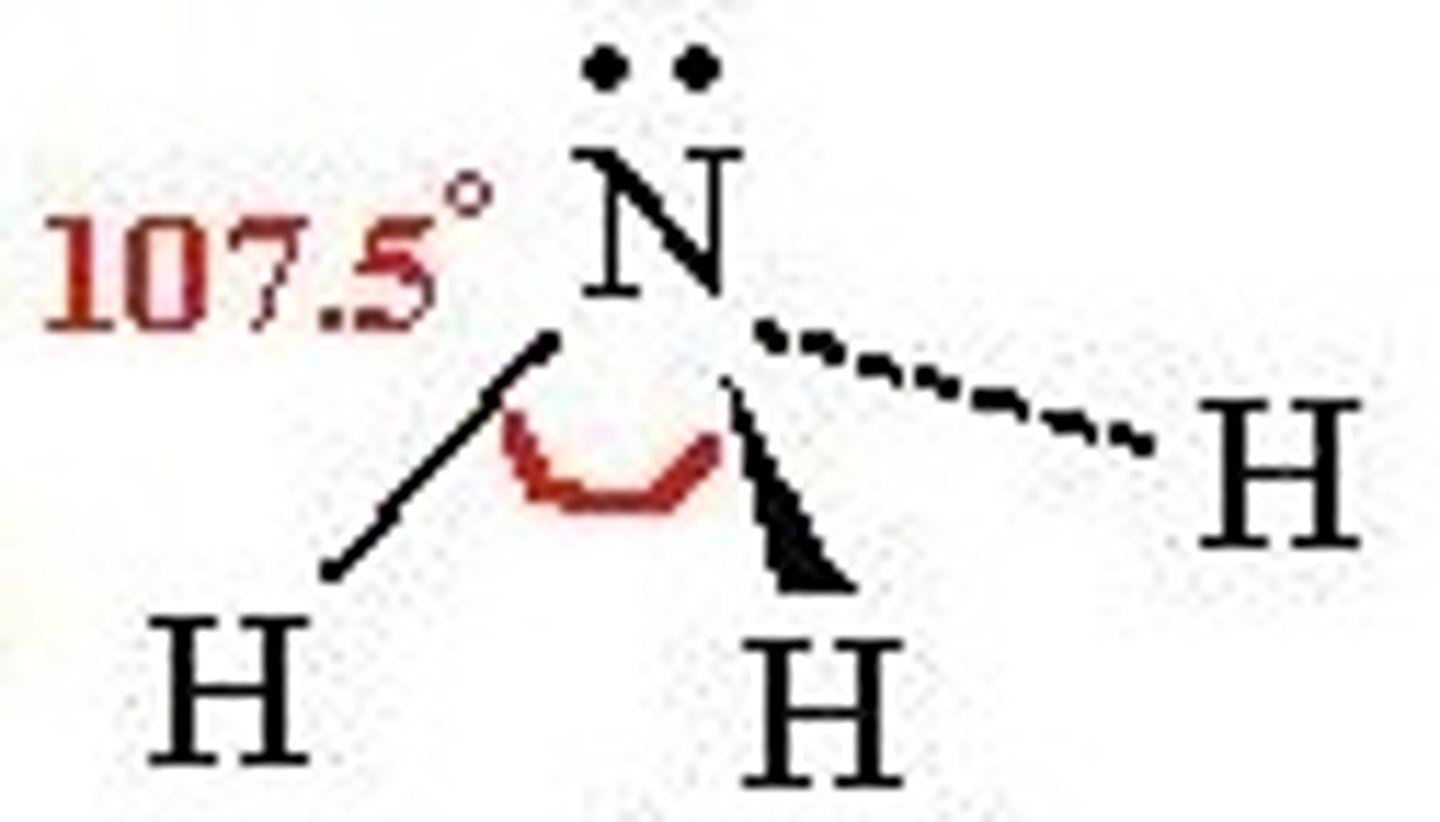
what shape is this?
pyramidal =107.5
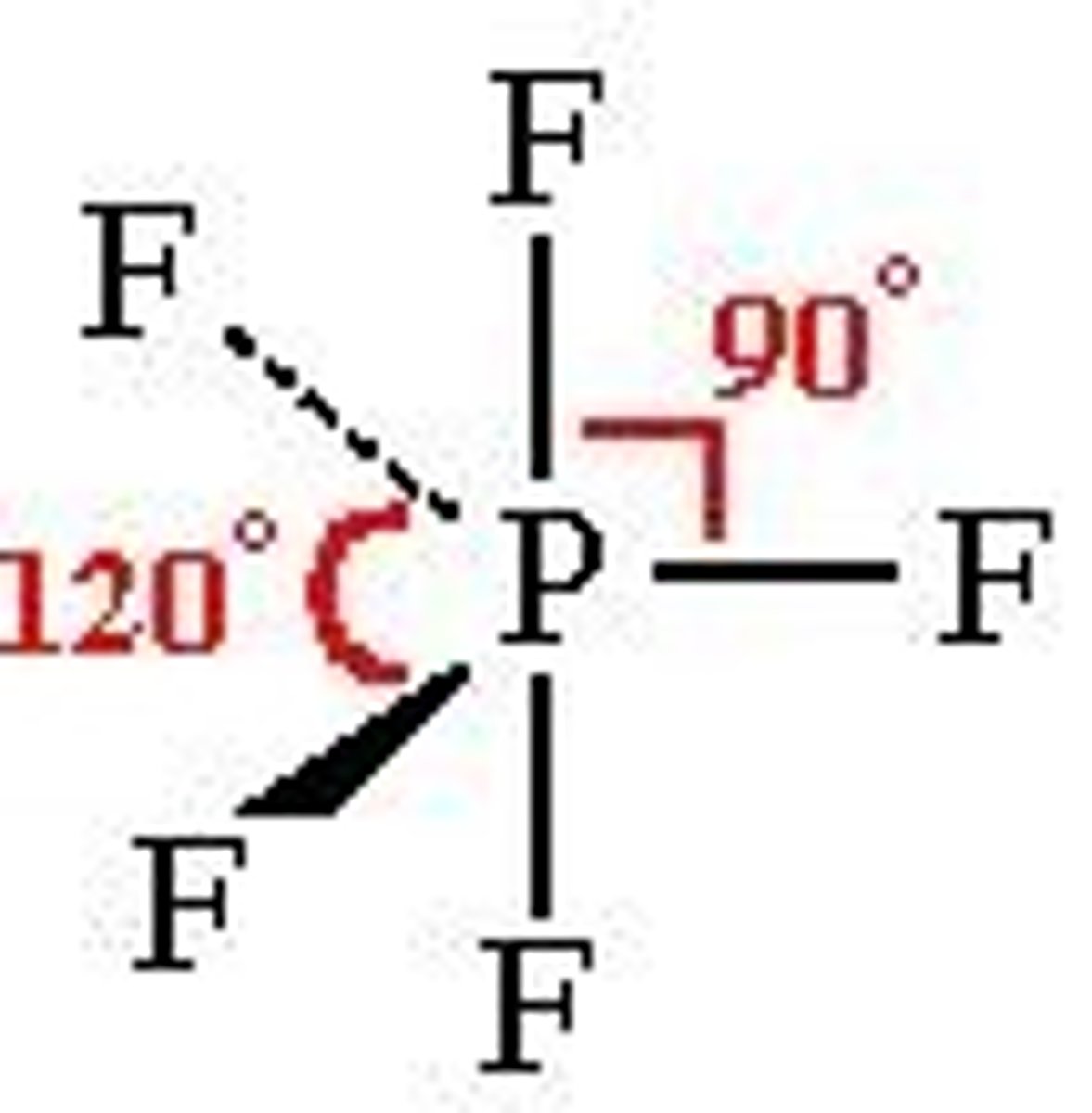
what shape is this?
trigonal bipyramidal =120 and 90
what shape is this?
octrahedral =90
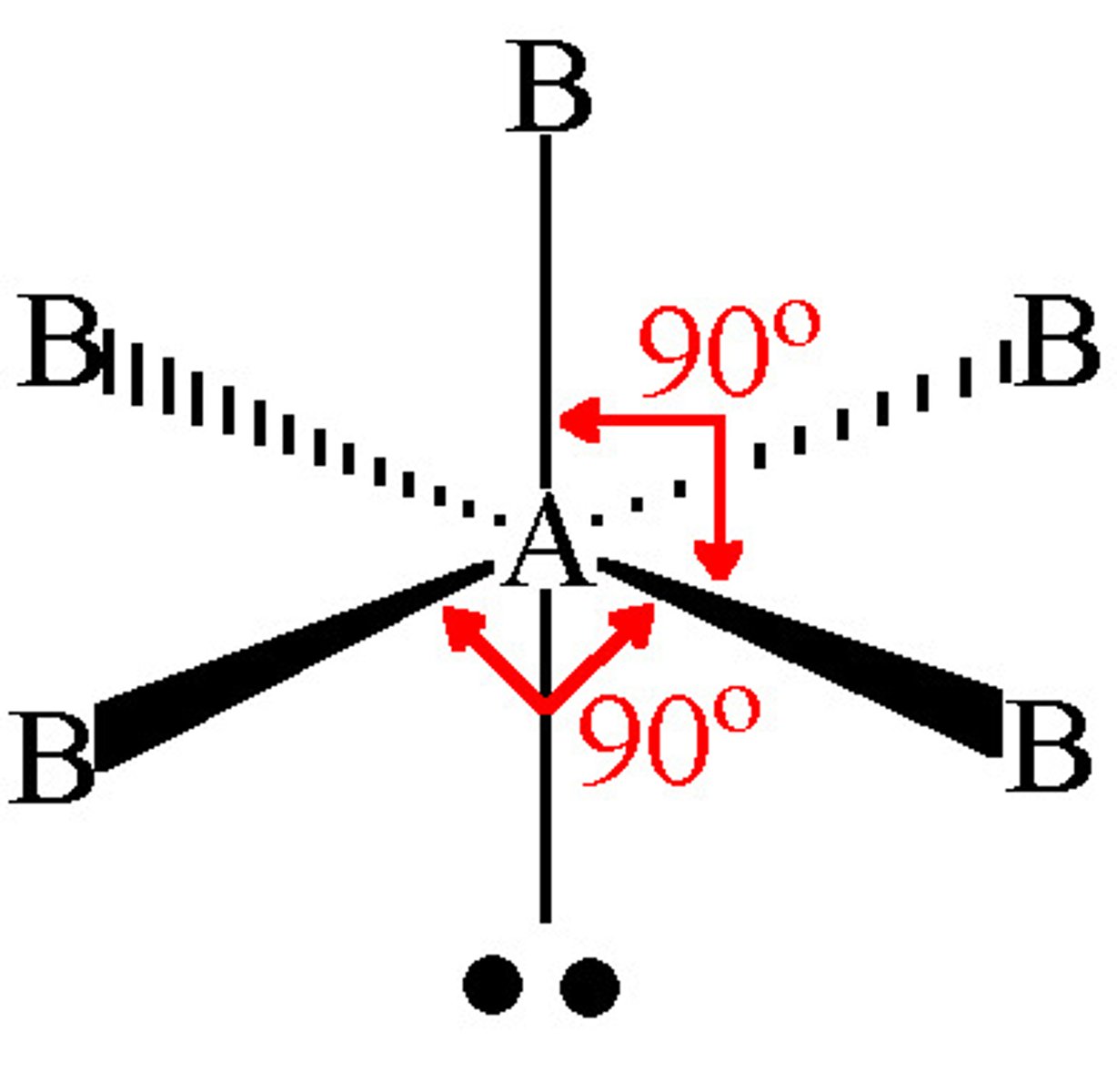
what shape is this?
square planar =90
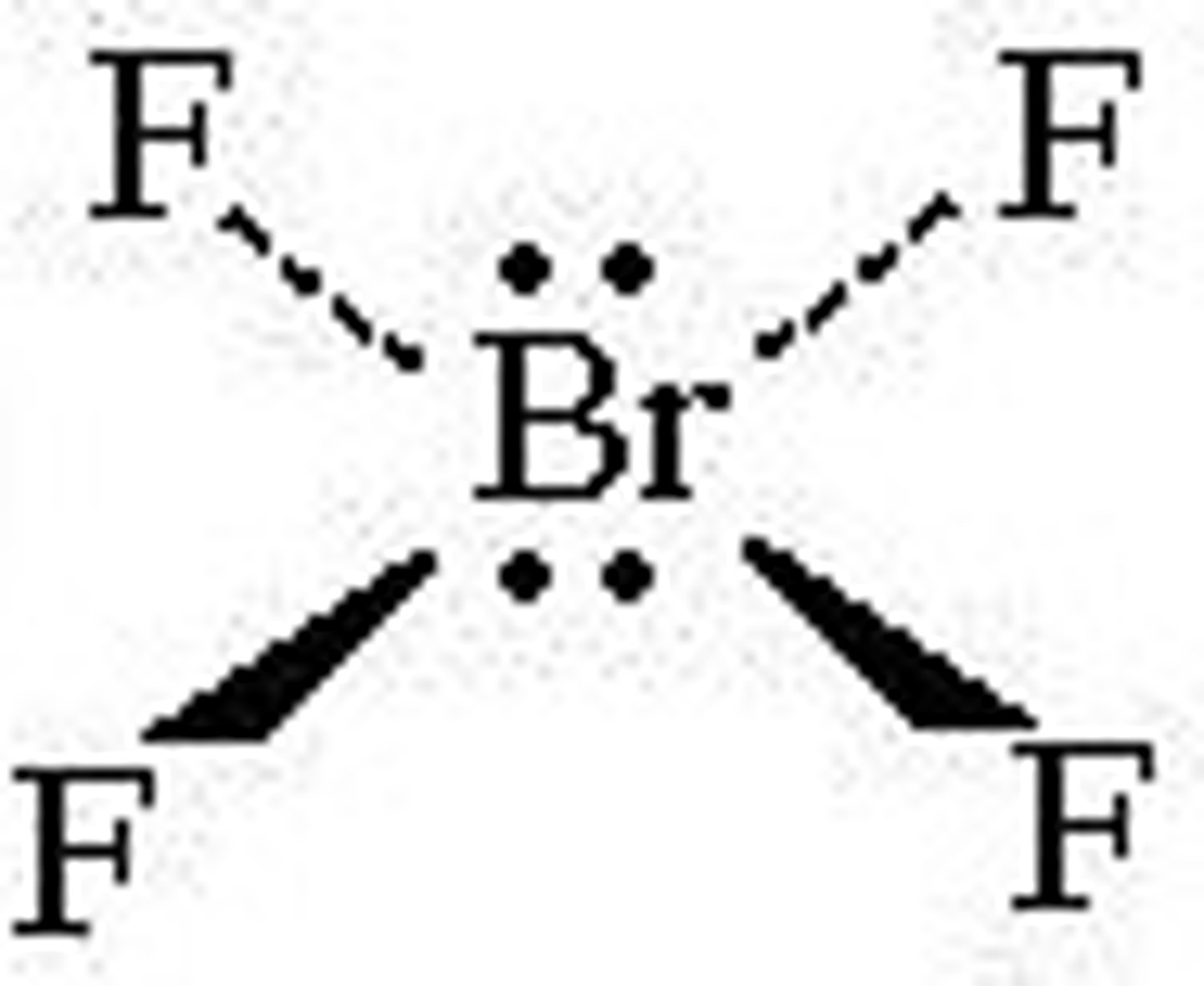
sp3 always makes what shape?
tetrahedral
octrahedral is
d2 sp3