Berkeley Chem 1A midterm 2 review
1/39
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
40 Terms
do we need more energy to heat more water of the same amount of energy?
more energy! we need more energy to heat more water because we need more energy to raise the temperature (kinetic energy) of more water because there are more water molecules
Heat capacity
usually in J/mol*C depends on amount of what you are calculating the heat capacity for
specific heat
usually in J/g*c does not depend on amount
Kinetic energy in relation to temperature
changes in Kinetic energy are changes in temperature. High kinetic energy means high temp
Endothermic
a chemical reaction that absorbs heat (requires energy input) so the reaction feels cold to the touch when, q is positive, going from low KE to high KE, energy enters the system and exits the surroundings, (decreasing in stability)
Exothermic
the chemical reaction that produces heat is the reaction feels hot to the touch when, q is negative, going from high KE to Low KE, energy exits the system enters the surroundings, (increasing instability)
formula for The amount of heat gained or lost by a sample
q=MCΔT
q- usually given in KJ heat entering or exiting the system
M- mass (how much stuff) in moles (can be in grams)
C- specific heat in J/gC or J/molC
ΔT- change in Temp. given by (Tfinal-Tinitial)
Heat exchange between event and system/surroundings and system
qsurroudnings = -qsystem
When is stability achieved? for in KE
High kinetic energy: less stable
Low kinetic energy: more stable
low KE is more favorable/stable
Potential Energy (PE)
changes in potential energy are changes in position (does NOT rely on temperature as KE does!!!). potential energy has no fixed zero
when is stability achieved for potential enegry?
High potential energy: less stable (positive)
Low potential energy: more stable (negative)
low PE: more favorable/stable
when do we need enegry input for potential energy?
when potential energy is low, if we want to get from low to high potential energy we need the energy to be inputted (low PE means low movement)
Potential energy (liquid -----> gas)
liquid is low PE state and gas is High PE state energy must be inputted to make liquid a gas
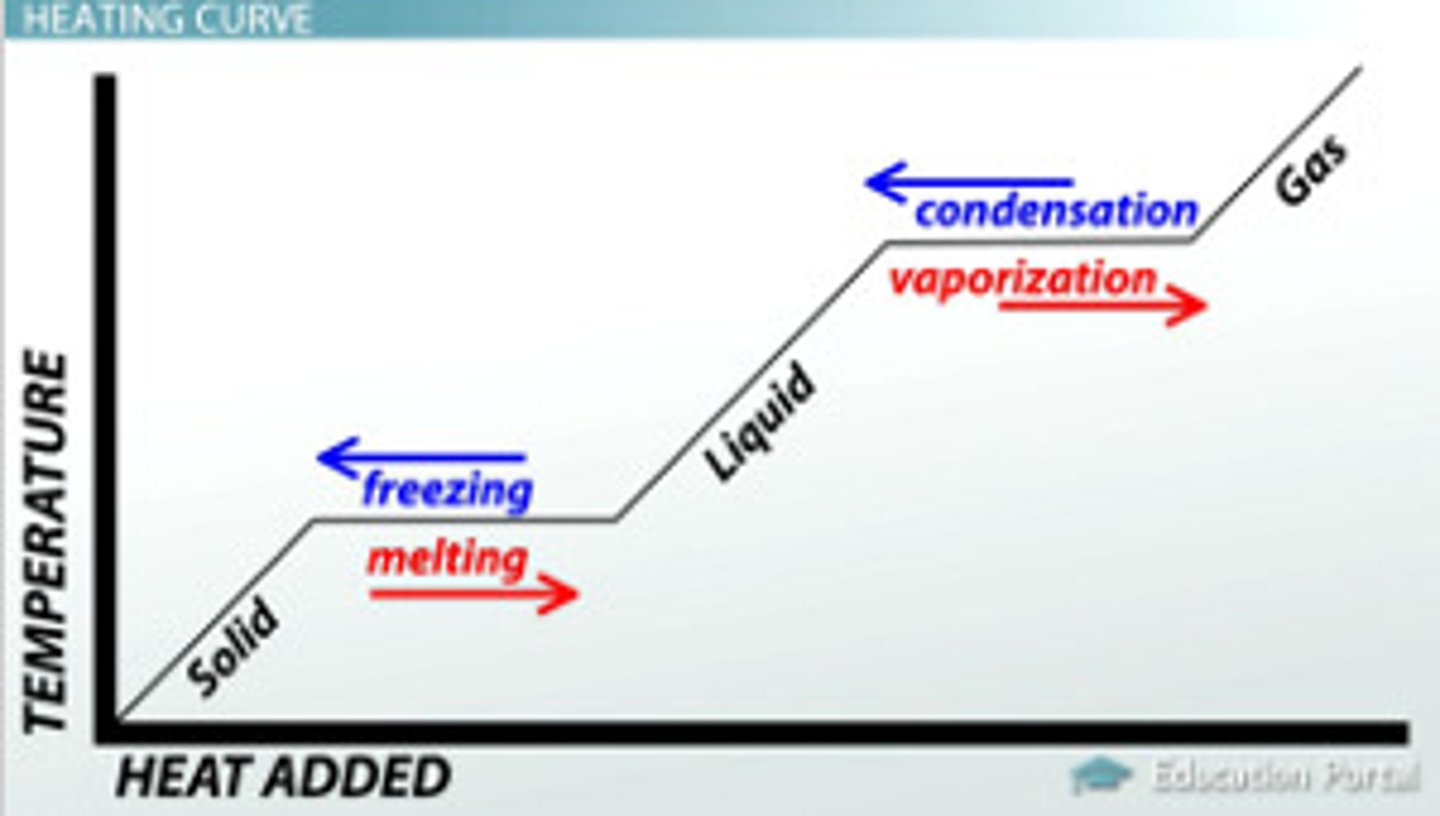
what is the relationship between Heat and phase changes
when heat is added to a solid temperature increases once a specific heat is reached the solid become gas (melting/fusion) (the reverse of this is freezing). As we add heat to a liquid the temperature increases once a specific heat is reached the liquid becomes a gas (boiling or evaporation) (the reverse of this is condensation)
ΔHevaporazation or ΔHvap
the energy required to boil/evaporate 1 mol , usually given in KJ/mol usually, a positive number initially because we need energy input for evaporation
the reverse is -ΔHcondense
ΔHfushion
the energy required to melt 1 mol, usually given in KJ/mol
usually, a positive number initially because we need energy input for fusion
the reverse is -ΔHfreeze
Chemical change
making or breaking chemical bonds
example: creating or breaking a covalent bonds, we would need to write the chemical formula differently
Physical change:
particles rearrange no bonds are made or broken
example: in boiling water, the molecules stay together but they are further apart from each other no bonds are broken or made os we do not have to change the chemical formula
Bond energy
the energy required to break a chemical bond is usually expressed as +KJ/mol (always positive, because we need energy INput to break bonds)
what does higher and lower bond order mean?
higher bond order means more energy is required to break all of those bonds and lower bond order means less energy is needed to break the bonds, so 1 bond requires less energy to break than 2 bonds and 3 bonds require less energy to break than 3 bonds etc.
example: c-c < c=c
bond energy and bond length relationship
As bond order increases (more bonds holding things together) the shorter the bond length with be distance of the things (relates to coulombs law)
change in enthalpy
written as ΔH, when is it negative it is exothermic (products are more stable/favorable) when it is positive it is endothermic (reactants are more stable)
we can find ΔHrxn by doing:
ΔHproducts-ΔHreactants
oxidation transfer
species loses the electron to another species
reduction
species gains the electron from another species
change in entropy
written as ΔS, when it is negative it is decreasing in entropy (less favorable) when it is positive it is increasing in entropy (favorable)
we can find ΔS by doing ΔS=Δh/T
is hard to calculate if
T is also changing, so best to use it
during phase transitions when T is
NOT changing
we can find ΔSrxn by doing:
ΔSproducts-ΔSreactants
phase changes and entropy
the higher the entropy (randomness) the more favorable a reaction: gasses = the most favorable (the most random) liquids = the second most favorable and solids = the least favorable phase. if we go from less favorable (s) or (l) to more favorable (g) the reaction is spontaneous(favorable). if it is the opposite the reaction is not spontaneous (not favorable).
change in Gibbs free energy
written as ΔG, when it is negative it is favorable (more spontaneous product favored) when it is positive it is not spontaneous (not favorable reactant favored)
we can find it by doing ΔH° - TΔS°
reaction beginning with only products
we can only go backwards (make more reactants) because if we only have products we need reactants
reaction beginning with only reactants
we can only go forwards (make more products) because if we only have reactants we need products
Using Hess's law to determine enthalpy changes from enthalpy changes of formation
we can use Hess's law to manipulate multiple reactions to create 1 reaction that we want, keep in mind when we manipulate reactions we must do the same to the heat of the reaction
ΔG^0cell (delta Gnot of the galvanic cell)
favorable when less than 0 (-) found by using the Nernst equation ΔG^0cell=-nFΔE^0cell
n= number of mols of electros
F= 96.5kj/molV (faraday's constant)
or we can find it by using Hess's law method
ΔE^0cell (delta Enot)
favorable when greater than 0 (+) found by
subtracting the ΔE^0 of the reduction and ΔE^0 of the oxidation
or we can use Hess's law method
Galvanic cell
reduction and oxidation reactions in separate locations with a salt bridge (two half cells)
salt bridge
A tube that allows the slow transfer of ions and maintains the neutrality of the solutions. Spectator ions travel through salt bridge and not electrons.
cathode
the half cell where the reduction occurs specifically in the metal electrode. we can tell if it is the cathode if it has the higher ΔE^0 value (more favorable)
anode
the half cell where the oxidation occurs specifically in the metal electrode we can tell if it is the anode if it has the lower ΔE^0 value (less favorable)
ΔG^0 (delta g not)
not to be confused with ΔG!
ΔG^0 is the change is Gibbs free energy at standard conditions products are not favored and reactants are not favored
ΔG=-TΔstotal
converting from the second law of thermodynamics to Gibbs free energy, using this equation we can tell if a reaction is favorable or not, recall that ΔG is favorable when it is negative
ΔGrxn
we can calculate this by doing the equation
ΔGrxn=ΔHrxn-TΔSrxn
when is a reaction spontenous and at what temperatures?
when ΔS>0 and ΔH<0 is spontaneous at all T (g>0)
when ΔS>0 and ΔH>0 is spontaneous at high temperatures (when TΔS is large)
when ΔS<0 and ΔH<0 it i spontaneous at low temp (when TΔS is small)
when ΔS<0 and ΔH>0 is it non spontaneous at all T (g<0)