3. Pluripotent stem cells and differentiation
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
Why are embryos and their stem cells difficult to study in vivo, and what solution does in vitro modeling provide?
Studying development *in vivo* (within a living organism) presents several major challenges:
Small cell numbers: The early embryo contains very few cells, making molecular and biochemical analysis difficult.
Inaccessibility: Development occurs in utero, making direct observation and manipulation of the embryo complex and invasive.
Ethics: There are significant ethical restrictions on the use of human embryos for research.
Solution: The capture and culture of pluripotent stem cells (PSCs) in vitro (in a petri dish) provides a powerful solution. These cells can be expanded to large numbers, allowing for detailed study. They can then be differentiated to model embryonic processes, providing an accessible and ethically more tractable system to understand development and disease.
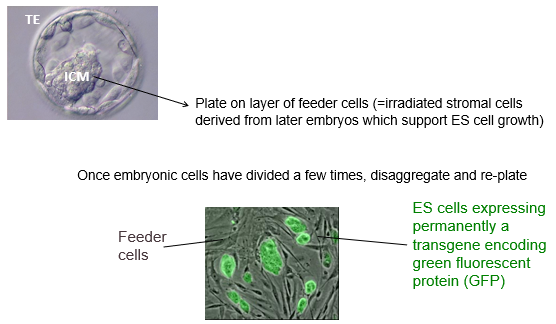
How are embryonic stem (ES) cells derived from a blastocyst and maintained in an undifferentiated state in vitro?
The process involves:
Isolation: The Inner Cell Mass (ICM) is surgically or immunologically isolated from a blastocyst.
Plating: The ICM is plated onto a layer of "feeder cells." These are typically irradiated mouse embryonic stromal cells (e.g fibroblasts) that secrete essential growth factors to support ES cell survival and growth.
Expansion: The ES cells divide and form colonies. These colonies are then disaggregated (broken up) and re-plated to expand the culture.
To maintain the cells in a self-renewing, undifferentiated (pluripotent) state without feeders, specific critical signals are added to the culture medium. The key signals differ between species:
Mouse ES cells: Require LIF (Leukaemia Inhibitory Factor) and BMP.
Human ES cells: Require FGF2 and TGFβ.
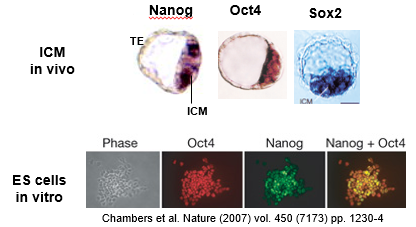
What are the key lines of evidence that demonstrate that embryonic stem (ES) cells cultured in vitro are truly pluripotent?
The pluripotency of ES cells is confirmed by several key observations that show they are equivalent to the *in vivo* Inner Cell Mass (ICM):
Gene Expression: They express the core pluripotency transcription factors Oct4, Nanog, and Sox2, and do not express genes indicative of differentiated lineages.
Self-Renewal: A single ES cell can divide to generate two identical, undifferentiated daughter stem cells.
Teratoma Formation: When transplanted into an immunocompromised mouse, ES cells form teratomas—benign tumours containing tissues from all three germ layers (ectoderm, mesoderm, endoderm). This is the "gold standard" functional test.
Chimera Formation: The most stringent test involves injecting ES cells (e.g., expressing GFP) into a host blastocyst. The injected cells integrate into the host embryo and contribute to all tissues of the resulting chimeric animal, proving their ability to participate in normal development.
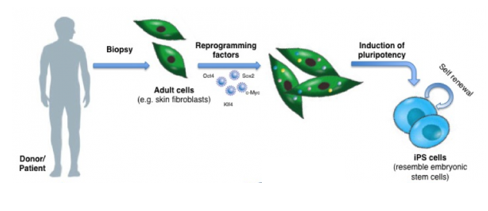
What are induced Pluripotent Stem (iPS) cells, and how did their discovery revolutionize stem cell biology?
Induced Pluripotent Stem (iPS) cells are adult somatic cells (like skin fibroblasts) that have been artificially reprogrammed back to a pluripotent state, making them functionally equivalent to embryonic stem (ES) cells.
It was discovered that introducing just four key transcription factors—Oct4, Sox2, Klf4, and c-Myc (often called the "Yamanaka factors")—was sufficient to erase the epigenetic memory of a differentiated cell and induce pluripotency.
The discovery was revolutionary because it allows for the creation of patient-specific pluripotent stem cells without the use of embryos, bypassing major ethical concerns and opening the door to personalized disease modeling and cell replacement therapies.
What is the general principle of directed differentiation, and what are the two main approaches used to guide pluripotent stem cells (PSCs) into specialized cell types in vitro?
The general principle of directed differentiation is to recapitulate normal embryonic development in a dish. This is done by removing the signals that maintain pluripotency and self-renewal, and then providing specific signaling molecules (like WNT, FGF, BMP) in a precise sequence and concentration to guide the PSCs towards a desired specialised cell fate.
There are two main approaches:
3D Differentiation (e.g., Embryoid Bodies, Organoids): Cells are grown in suspension, allowing them to self-assemble into 3D aggregates. This more accurately mimics the embryonic environment but makes it difficult to dissect the role of individual signals.
2D Differentiation (Adherent Culture): Cells are grown as a monolayer on a specific substrate. This is a more tractable system for live imaging and testing specific signals but loses complex 3D cell-cell interactions.
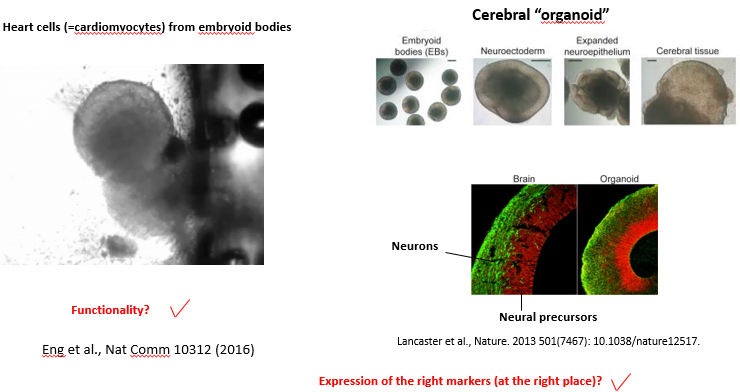
Describe the 3D approach to in vitro differentiation and provide examples of its application. What are its main advantages and disadvantages?
The 3D approach involves removing self-renewal signals (e.g LIF/BMP for mouse ES cells and FGF2/TGFb for human ES cells) and allowing pluripotent stem cells to aggregate in suspension. These aggregates, called Embryoid Bodies, spontaneously differentiate and begin to form derivatives of all three germ layers.
With more advanced protocols, the cells can be guided to form organoids, which are complex, self-organizing 3D structures that mimic the architecture and cell types of an organ.
Examples: Beating heart cells (cardiomyocytes) can be seen in embryoid bodies. Cerebral organoids can be generated that develop layered structures resembling the human brain cortex, with distinct zones of neural precursors and mature neurons.
Advantage: This method more accurately recapitulates the complex cell-cell interactions and 3D architecture of the *in vivo* embryonic environment.
Disadvantage: It is often difficult to precisely control and observe the process, or to dissect the specific role of individual signaling molecules.
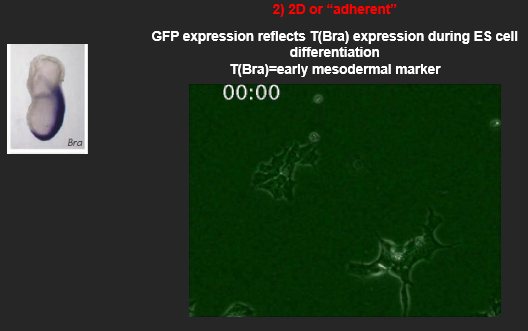
Describe the 2D (adherent) approach to in vitro differentiation. What are its main advantages and disadvantages?
The 2D approach involves a more controlled, step-wise differentiation protocol.
A defined number of cells are plated as a monolayer on a specific substrate/extracellular matrix.
Self-renewal signals are removed (e.g. BMP/LIF for mouse ES cells or FGF2, TGFb for human ES cells).
A defined medium containing a precise cocktail of signaling molecules (e.g., specific concentrations of FGF, WNT, BMP) is added to direct the cells towards a specific lineage.
Example: Using this method, one can track the expression of early mesodermal markers like T(Brachyury) using a GFP reporter, allowing for real-time visualization of differentiation.
Advantage: It is a more tractable and defined system, which makes it easier to test the role of specific signals and is well-suited for high-throughput screening and live-cell imaging.
Disadvantage: It lacks the complex 3D cell-cell interactions and architectural cues that occur *in vivo*, which may limit the maturation or function of the resulting cells.
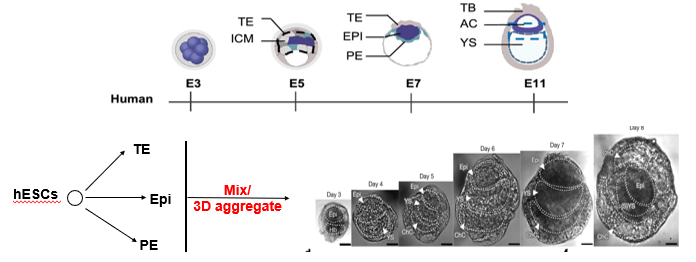
What are "synthetic embryos" and how do they represent a hybrid approach to in vitro differentiation?
"Synthetic embryos," also known as blastoids or stem-cell-based embryo models, are advanced *in vitro* structures that aim to recapitulate the architecture of the early post-implantation embryo. They represent a hybrid (2D/3D) approach.
They are generated by mixing 3 distinct types of stem cells that correspond to the three founding lineages of the blastocyst:
Embryonic Stem Cells (ESCs) → epiblast (future embryo).
Trophectoderm Stem (TS) Cells → trophectoderm (future placenta).
Extraembryonic Endoderm (XEN) Cells → primitive endoderm (future yolk sac).
When mixed in a 3D aggregate, these cells self-organize, demonstrating a remarkable ability to mimic the key morphogenetic events of early development, such as the formation of an epiblast cavity and extraembryonic tissues.
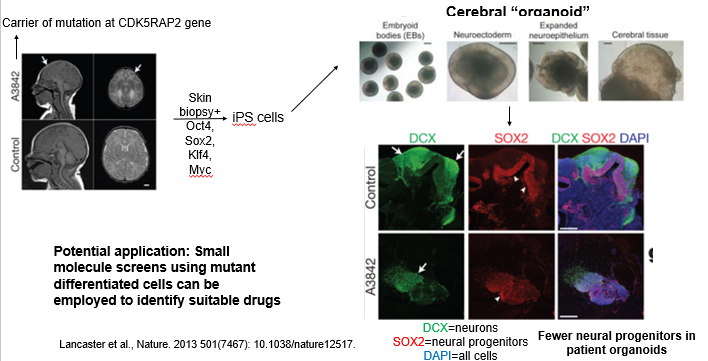
What is microcephaly and why would it need to be modelled using in vitro cell engineering?
How are iPSCs used for in vitro disease modeling? Use the case study of microcephaly to explain the workflow and its potential applications.
Microcephaly is a neurodevelopmental disorder caused by various autosomal recessive mutations (e.g in the CDK5RAP2 gene), infants are born with an abnormally small brain and it leads to neurological defects and seizures. Mouse mutants have failed to recapitulate the condition. Here is an example of using iPSCs to provide a powerful "disease-in-a-dish" platform.
The workflow is:
Biopsy & Reprogramming: Take a skin biopsy from a patient with a known genetic disorder (e.g., a mutation in the CDK5RAP2 gene causing microcephaly). Reprogram these cells into patient-specific iPSCs using reprogramming factors (aka the Yamanaka factors (Oct4, Sox2, Klf4, c-Myc)).
Differentiation: Differentiate both patient and healthy control iPSCs into the affected cell type. For microcephaly, this was done by generating cerebral organoids.
Phenotype Analysis: Compare the patient-derived model to the control. In the microcephaly study, the patient organoids were smaller and had significantly fewer neural progenitors (SOX2+ cells), successfully recapitulating the key disease phenotype.
Application: These patient-specific models can be used in high-throughput screens to test thousands of small molecules to identify drugs that can correct the cellular defect, paving the way for personalized medicine.
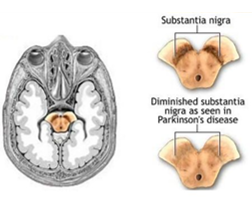
What is the principle of cell replacement therapy? Use the case study of Parkinson's disease to explain the strategy and its progress.
Cell replacement therapy aims to treat diseases caused by the death or dysfunction of a specific cell type by replacing them with healthy, functional cells grown in the lab.
Case Study: Parkinson's Disease
Pathology: Parkinson's is a neurodegenerative disorder caused by the progressive loss of midbrain dopaminergic (mDA) neurons in the substantia nigra. This leads to symptoms like tremor, rigidity, and slowness of movement.
Strategy:
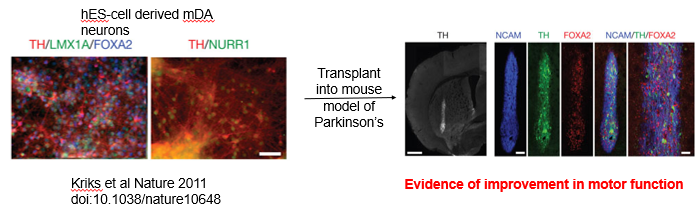
Differentiate human pluripotent stem cells (hPSCs) *in vitro* into pure, functional mDA neurons (expressing key markers like Tyrosine Hydroxylase (TH), LMX1A, and FOXA2).
Transplant these lab-grown neurons into the brains of patients.
Progress: This approach has shown great promise in animal models of Parkinson's, with transplanted neurons surviving, integrating, and leading to significant improvement in motor function. This has progressed to Phase I clinical trials in human patients.
Can we capture later stem cell/progenitor populations in vitro?
How can multipotent stem cells, such as Neural Stem Cells (NSCs), be captured and maintained in vitro?
Yes, later-stage multipotent stem cells can be isolated from tissues and cultured *in vitro*. The strategy involves providing a specific environment that maintains their "stemness" and prevents differentiation.
Example: Capturing Neural Stem Cells (NSCs) which are bipotent
Source: Dissociate tissue from the fetal brain.
Culture Conditions: Plate the cells on a laminin substrate in the presence of the cytokines FGF2 and EGF. These signals act as the "niche" factors that promote NSC self-renewal and proliferation while keeping them in an undifferentiated state. The NSCs express undifferentiated markers (e.g RC2) but not have no expression of genes indicative of differentiation (Glia = GFAP+, Neuron = TUJ1+).
Proof of Potency: To confirm they are true stem cells, FGF2 and EGF are removed. In the absence of these niche signals, the NSCs will spontaneously differentiate into both neurons and glia (astrocytes/oligodendrocytes), demonstrating their bipotency.
