Kidney disease
1/50
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
51 Terms
Urinary system:
Kidneys x 2
Urinary bladder – temporary storage for urine
Ureter x 2 – urine transport: kidney to bladder
Urethra – urine from bladder to external
Functions of the kidney
Excretory functions
metabolic wastes: urea, creatinine
Excretion of bioactive substances (hormones, foreign substances, drugs)
Toxins
Endocrine functions
Erythropoietin
Renin
Prostaglandins
Regulatory functions
Water balance
Electrolyte balance & acid-base balance
Sodium
Potassium
Chloride
Bicarbonate
Calcium
Magnesium
Metabolic functions
Vitamin D (1 alpha,25 dihydroxy cholecalciferol)
Vitamin D is produced from the skin
The kidney
Kidneys-Two sections
Outer cortex
Inner medulla
The functional unit of the kidney is Nephron
Approximately one million in each kidney
capable of forming urine
Two major components:
Glomerulus
Tubular system
Proximal tubule
Diluting segment
Distal tubule
Collecting duct
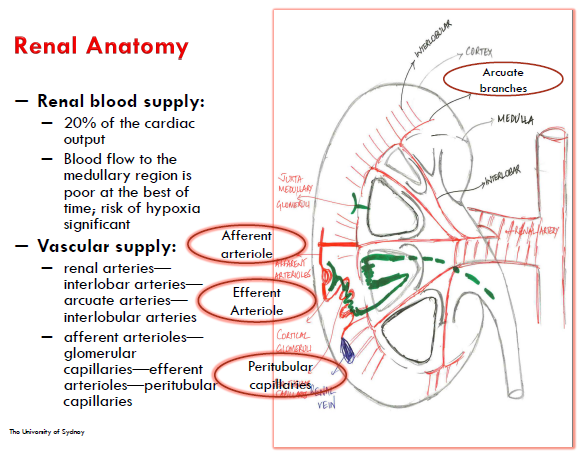
Renal anatomy
Renal physiology
Steps in urine formation
Filtration:
Filtration only takes place in the renal corpuscle.
180 litres of fluid is filtered by the nephrons in one day!!
Reabsorption
Reabsorption occurs when filtered material is moved back into the blood,
Secretion
while secretion removes selected material from the blood and places it in the filtrate.
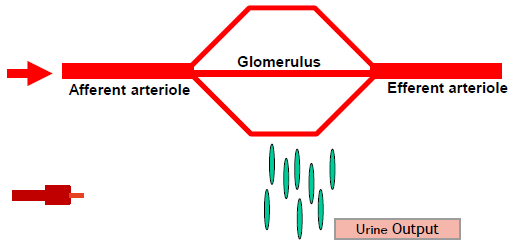
Filtration (golmerular function)
The glomerulus is a high-pressure filtration system composed of a specialized capillary network.
Blood supplied to the glomerulus through the Afferent Arterioles (AA) and removed by the Efferent Arterioles (EA)
Large molecules are generally unable to pass through the glomerular membranes
It generates an ultra filtrate that is free of blood and significant amounts of blood proteins.
Ultra filtrate passed into the tubular system for processing eg reabsorption of water & electrolytes, regulation of concentration, etc
Glomerular filtration rate (GFR) is a key marker of renal function
Index of renal function would thus ideally measure GFR
Physiology of renal blood flow
GFR usually maintained by factors such as cardiac output, SNS tone, blood pressure, vascular volume, etc
Reduction in any of these drivers will lead to reduced GFR and hence urine production
A feedback mechanism that keeps renal blood flow (RBF) and GFR constant despite changes in arterial blood pressure.
As RBF increases, GFR increases, leading to an increase in NaCl delivery to the macula densa.
a feedback loop through the macula densa to the juxtaglomerular cells of the afferent arteriole results in increased vascular tone, decreased renal blood flow and a decrease in GFR.
NaCl to the macula densa then decreases leading to relaxation of the afferent arteriole (increasing glomerular hydrostatic pressure) and increases renin release from juxtaglomerular cells of afferent and efferent arterioles
renin increases angiotensin I, then converted to angiotensin II which constrict efferent arteriole increasing hydrostatic pressure returning GFR to normal
The kidneys have inbuilt physiological defense measures to counteract reduced inflow using Prostaglandins and Angiotensin II
In conditions of stress to the kidneys (eg CKD, DM, HTN, CCF) these mechanisms become increasingly important to maintain GFR.
Assessment of kidney function
Glomerular filtration rate (GFR): is the rate (volume per unit of time) at which ultra filtrate is formed at the glomerulus.
GFR primary measure of kidney function & hence critical knowledge to evaluate drug dosage
Normal is 100 to 120 ml/min
Filtration markers:
Exogenous (e.g. Inulin)
Endogenous (e.g. Serum Creatinine, Urea)
Endogenous markers
Urea:
End product of protein and amino acid catabolism
serum urea concentrations are influenced by both rate of protein breakdown and renal urea excretion
Filtered at glomerulus & reabsorbed in tubules (40-60%)
A relatively insensitive marker of renal function
Usually measured along with serum creatinine
Creatinine:
Waste product of muscle metabolism
formed by the liver via breakdown of creatine (from muscle)
usually produced at a constant rate dependent on muscle mass
Excreted by kidney - glomerular filtration (10% via secretion)
SCr is both a reflection of both muscle mass and kidney function
SCr is inversely proportional to glomerular filtration rate
doubling of SCr (even within the reference range) represents a 50% reduction in renal function
Good indicator of renal function (better than urea)
Drug dosing in kidney disease
GFR is the key clinical measure of kidney function.
In general, for drugs that are excreted by the kidney, a decrease in GFR is associated with a decrease in drug clearance and the dosage needs to be reduced.
The GFR can be quantitated in multiple ways.
Each has advantages and disadvantages.
The measured GFR (mGFR) is the gold standard, but it is resource intensive and expensive.
So, the estimated GFR (eGFR) is used to classify and monitor the severity of chronic kidney disease
This adjustment depends on the severity of the disease and what proportion of the drug is eliminated by the kidneys.
The estimated glomerular filtration rate can generally be used to guide dose adjustment in patients with stable kidney function.
However, the formula can be misleading in some patient subsets and other approaches are required.
At extremes of body mass, the estimated glomerular filtration rate can under- or overestimate kidney function. It may need to be adjusted for body surface area, particularly for drugs with a narrow therapeutic range.
Serum creatinine-based formulae
GFR can be assessed using serum creatinine-based formulae – Cockcroft-Gault and CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration).
IBW:
men: 50 + (0.9 x height cm> 152cm)
women: 45 + (0.9 x height cm> 152cm)
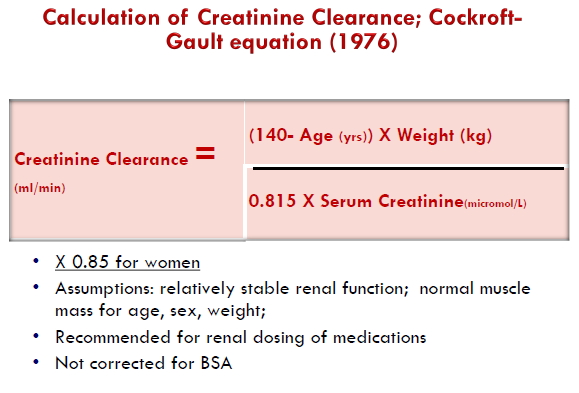
CG formula
CG Formula is not routinely used currently
Because eCrCl was validated against measured CrCl based on 24-hour urine collection, it overestimates the actual GFR given that creatinine is both filtered and secreted in the nephron tubules.
By 2010 most laboratories in Australia were using a newer creatinine assay standardised to isotope dilution mass spectrometry (IDMS) which resulted in a 10–20% decrease in creatinine concentrations.
Therefore, there will increase the eCrCl compared to what would have been calculated pre-2010.
May be recommended for some select drugs (e.g. Dabigatr
eGFR-CKD-EPI
The CKD-EPI formula estimates GFR because it was validated against GFR measured using exogenous filtration markers.
It incorporates age and sex into a relatively complicated formula.
eGFR is automatically calculated and reported by the laboratory.
The units for the automated eGFR are mL/min/1.73 m2
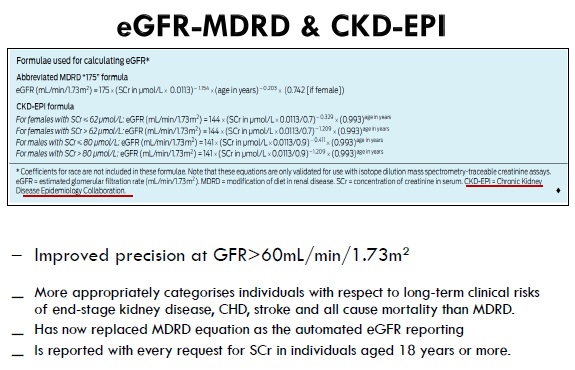
Which formula to use for drug dosing
Important to note neither is a perfect representation of the true value of the GFR.
Second, the eCrCl and automated eGFR do not give exactly the same results and eCrCl generally overestimates mGFR.
For patients with a body surface area that is substantially different from 1.73 m2, the eGFR can be de-indexed to give units of mL/minute
Drug dosage adjustment in kidney impairment
eGFR provides a valid estimate of kidney drug clearance and is widely available on laboratory reports.
For most drugs in clinical practice eGFR vs CG equations has no major difference in dosage recommendations
Caution is advised in special groups (amputees, obesity, pregnancy) or when dealing with narrow therapeutic index drugs
Depends upon proportion of drug eliminated by the kidney, risk of adverse effects, duration, and if the drug has active or toxic metabolites that rely on kidney for elimination
Dose adjustment in kidney impairment usually involves increasing dosing interval or reducing dose
Important to focus on the patient-related, disease-related and medication-related characteristics and use clinical judgment
Drug dosing consideration in AKI
Optimising drug therapy for patients with acute kidney injury (AKI) is challenging
Factors that need considerations include; residual drug clearance, accumulation of fluids, and dialysis.
For renally cleared drugs (>30% elimination unchanged in the urine), particularly for drugs with narrow therapeutic range, serum drug concentration and pharmacodynamic response is necessary.
Acute kidney injury (AKI) (acute renal/kidney failure, ARF)
Abrupt decline in renal function leading to an increase in serum concentrations of urea, creatinine & other substances (occurs over a period of days)
Common - occurs in 2% to 7% of all hospital admissions
and in up to 36% to 67% of critically ill patients
very common amongst the elderly
may occur in someone either with previously normal renal function or as an acute and unanticipated deterioration in function in a patient with previously established chronic kidney disease (“acute on chronic”)
Major risk factors for acute kidney injury (AKI)
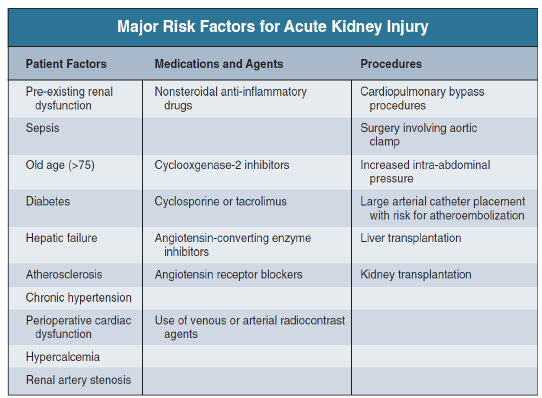
Stratification of AKI
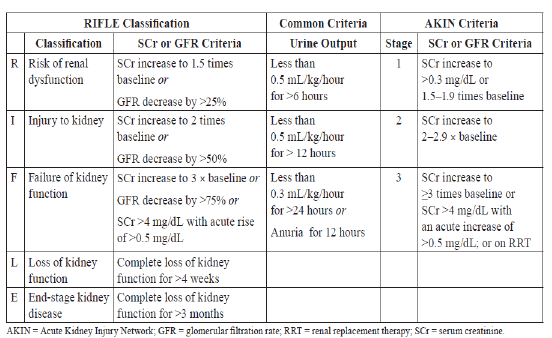
Diagnosis of AKI
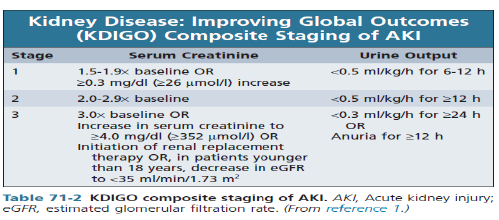
Types of AKI-pathophysiology
Three categories of AKI:
Pre-renal (40-80% of cases)
diseases characterized by renal hypo perfusion in which the integrity of renal parenchymal tissue is preserved
Intra-renal or intrinsic (10-50% of cases)
diseases involving renal parenchymal tissue
Post-renal or obstructive (<10% of cases)
diseases associated with acute obstruction of the urinary tract (i.e. ureters, urethra etc)
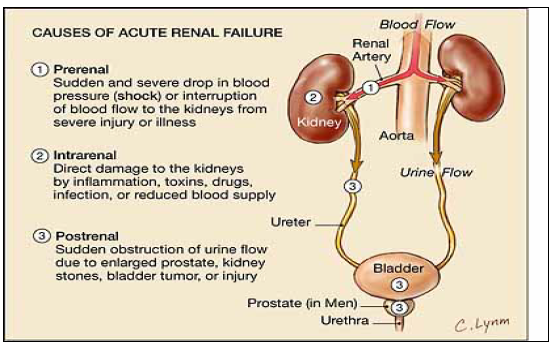
ACE I and NSAIDs
ACEI & ARBS block ATII which dilates the efferent arteriole and decreases glomerulus filtration pressure
NSAIS block prostaglandins which then constricts the afferent arteriole
Clinical manifestations
frequently occur late in the course and are often not apparent until renal dysfunction has become severe
early signs often non-specific and occur in addition to other clinical conditions
hypertension, oedema (esp. in patients who are fluid overloaded)
anorexia, fatigue, nausea and vomiting, and pruritis (uremia)
a decline in urine output or dark-coloured urine oliguria and/or anuria
Note: High mortality – non-oliguric AKI better prognosis
AKI: prevention (SADMANS)
Identification of high-risk individuals
Optimisation of renal perfusion
volume expansion and/or fluid therapy where appropriate
vasopressors (e.g. adrenaline, dopamine) may be used once intravascular volume has been restored (patchy evidence however)
Avoidance of nephrotoxins wherever possible (and close monitoring when not)
includes drugs, and combinations of drugs, e.g. triple whammy
Specific circumstances (examples)
once daily dosing of aminoglycosides
allopurinol and rasburicase to prevent tumour lysis syndrome
Amphotericin whenever possible; limiting dose, rate of infusion.
Recognise that patients with pre-existing renal impairment are at higher risk of developing further renal insufficiency—treat and monitor accordingly
Temporarily withhold nephrotoxins (especially ACE-I, ARBs, NSAIDs) and diuretics (to prevent dehydration) when patients become unwell—either in the community or in hospital
SADMANS group of drugs
Ensure that patients remain adequately hydrated, to maintain renal perfusion
Remember to monitor renal function after starting, or increasing the dose, of ACE-I or ARBs (check one to two weeks later)
Where necessary, adjust drug doses in patients with renal impairment
Monitor drug levels when using aminoglycoside (gentamicin) and/or glycopeptide (vancomycin) antibiotics - and adjust dose accordingly
Hydrate the patient and consider using N-acetyl cysteine before procedures entailing radiological contrast media

AKI: management
The most important step is to reverse the cause (which will depend on the type of AKI)
Pre-renal
improve perfusion, remove offending medications
Intrinsic
treatment of ATN primarily supportive
no specific therapeutic intervention has been found to hasten recovery of kidney function
use of diuretics to convert patients from oliguric to nonoliguric ATN not associated with improved outcomes
may be helpful in volume management (need high doses e.g. >160mg frusemide), but cease if no response
for AIN, avoid offending agent in future
Post-renal
relieve obstruction
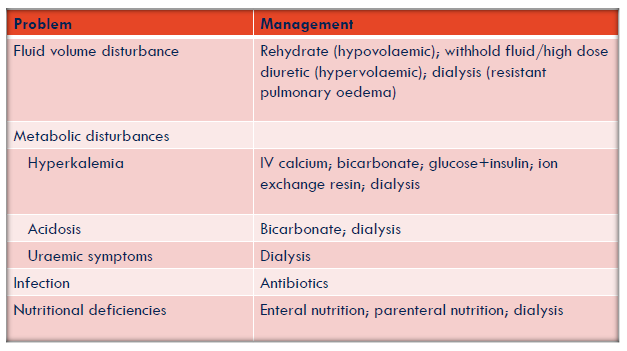
All patients with significant AKI also require attentive management of volume, electrolyte (esp K+) and acidbase status, and nutrition
Renal replacement therapy (RRT, dialysis) may be required in cases of severe AKI (hyperkalaemia, volume overload, severe acidosis, or overt uraemia)
Management of hyperkalaemia + other AKI complications
(blood potassium levels are too high and can cause life-threatening heart arrhythmias, muscle weakness, and paralysis)
Severe hyperkalaemia (K+ >6.5 mmol/L) is a medical emergency because of the risk of life threatening cardiac arrhythmias
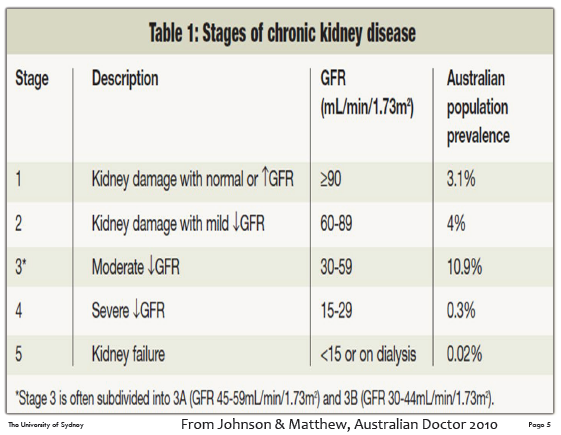
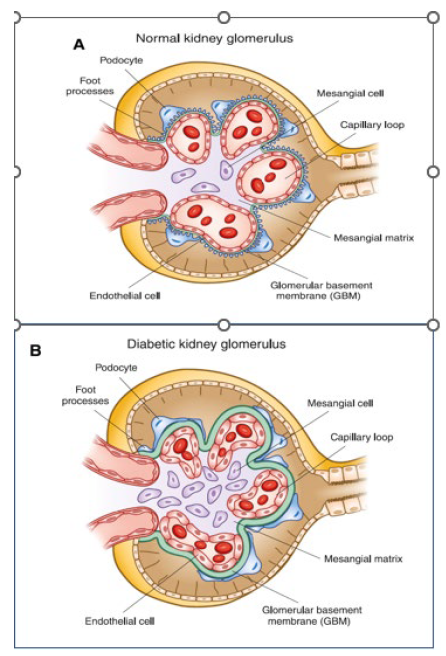
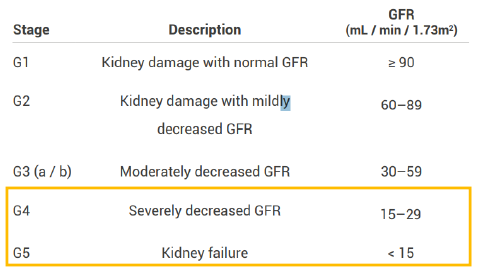
Chronic kidney disease (CKD)
CKD is defined as kidney damage or GFR below 60ml/min/1.73m2 for 3 months or more irrespective of the cause.
CKD is
a long-term health condition (months/years), preventable in many cases
glomerular & tubular damage
asymptomatic until much of the kidney function is lost
compensatory and adaptive mechanisms maintain acceptable health until GFR is ~10 to 15 ml/min
CKD: risk factors
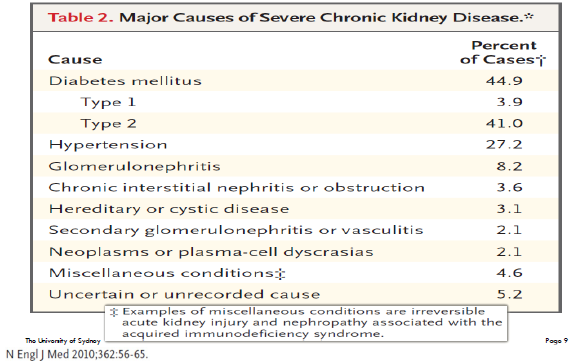
CKD: major causes
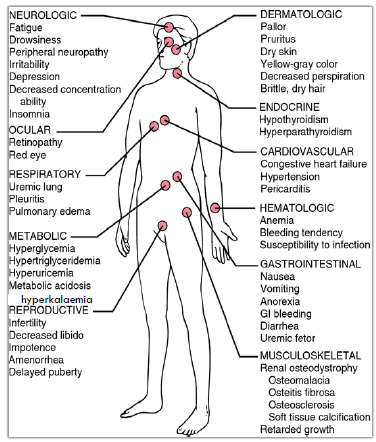
Clinical manifestations
CVD in patients with CKD
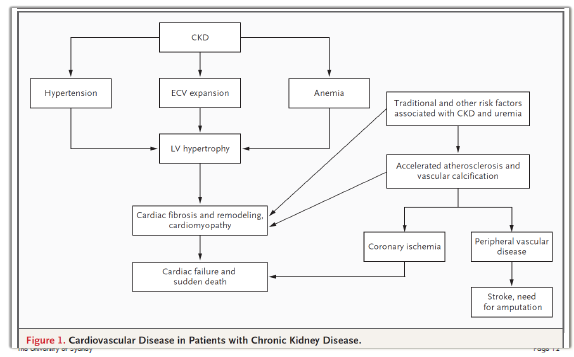
General Management
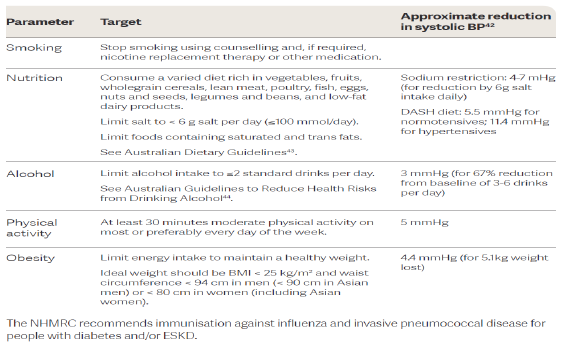
Management of CKD
Preserving renal function: hypertension
both symptomatic of advanced CKD and pathogenic
lowering BP delays the rate of progression
ACEIs/AT2RAs are generally the best agents (esp in diabetic nephropathy), CCB (dihydropyridine) are also preferred.
most patients will require at least 2 drugs, probably more
Key take home message: ACEis/ARBs are first line agents in patients with CKD but can also be nephrotoxic.
As long as kidney function does not deteriorate by more than 25% (eGFR) of baseline within 2 months of initiation they are ok to continue.
OR potassium does not exceed by 6mmol/L they are ok to continue. They are renoprotective in the long run
Preserving renal function: proteinuria/albuminuria
degree of proteinuria correlates with rate of progression , and is the most reliable prognostic factor in CKD
absence of significant proteinuria or remission indicates favourable prognosis
low-protein diets are not promoted (little benefit)- but avoid high protein diets (aim for 0.6-0.8g/kg/day)
Management:
ACEI/ARBs, reduce salt, SGLT2i, Finerenone
Lipids and CKD
CKD is associated commonly with substantial abnormalities of lipid metabolism, including increased LDL, TG, VLDL, and reduced levels of HDL cholesterol.
Dyslipidaemia is more severe in individuals with albuminuria
Management:
Evaluate lipid profile in newly diagnosed CKD
If aged ≥50 years with any stage of CKD (irrespective of lipid levels)
Statin if eGFR is > 60 mL/min/1.73m2
Statin/ezetimibe combination if eGFR is ≤ 60 mL/min/1.73m2
If aged < 50 years with any stage of CKD (irrespective of lipid levels):
Statin if presence of one or more of: coronary disease, previous ischaemic stroke, diabetes or estimated 10-year incidence of fatal or non-fatal myocardial infarction above 10%
Glucose control
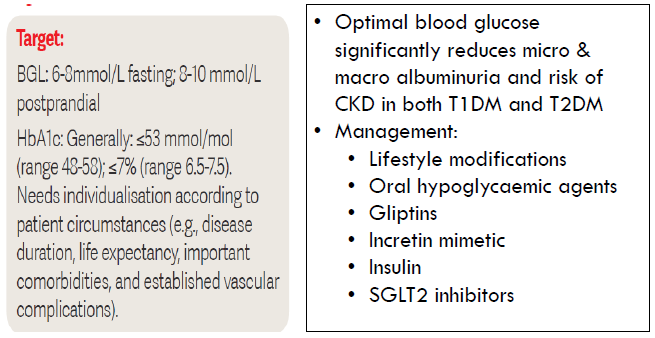
Four pillars of diabetic kidney disease
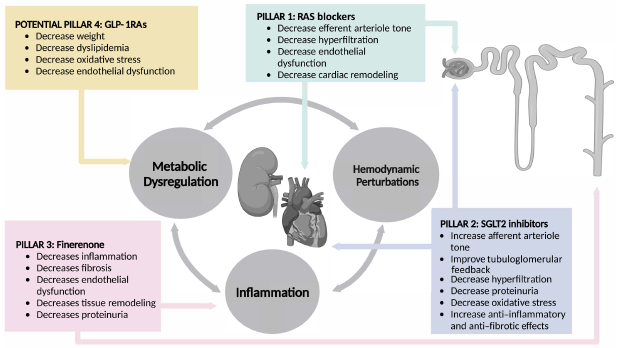
Four pillars of kidney disease cont…
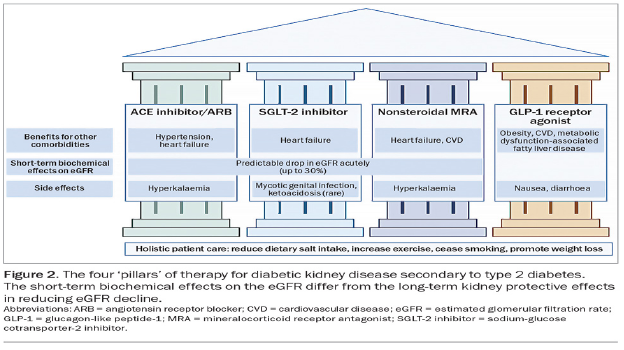
Metabolic acidosis
Kidney essential in maintaining acid–base balanc
usual symptom is SOBOE not explained by pulmonary oedema or anaemia
chronic acidosis in people with <30ml/min/1.73m2 also aggravates hyperkalaemia, inhibits protein anabolism & accelerates bone calcium loss and associated with increased morbidity
seldom requires treatment unless
[bicarb] ~<15 mmol/l, pH ~<7.30,
Sodium bicarbonate (SodiBic 840mg capsule) occasionally used
Typical starting dose 1 cap od/bd increasing up to 2 bd if needed to keep bicarb >22mmol/L
but carries a substantial sodium load
typically not required in dialysis
Hyperkalaemia management
If K+>7.0 mmol/L (3.5-5.0 mmol/L) → ECG changes → → cardiac arrest
Calcium gluconate 10% (10-20mL) IV → stabilises myocardium
Insulin (soluble) 10-20 units + glucose 50% (50mL) → stimulates K+ uptake into cells
Sodium (or calcium) resonium 15g TDS-QID po or enema → binds potassium & releases calcium
Chronic:
removal of drugs contributing to hyperkalaemia
reducing potassium dietary intake
Correct acidosis
increasing potassium elimination
K+ wasting diuretics (thiazides/loop)
cation exchange resins (polystyrene sulfonate, Resonium )
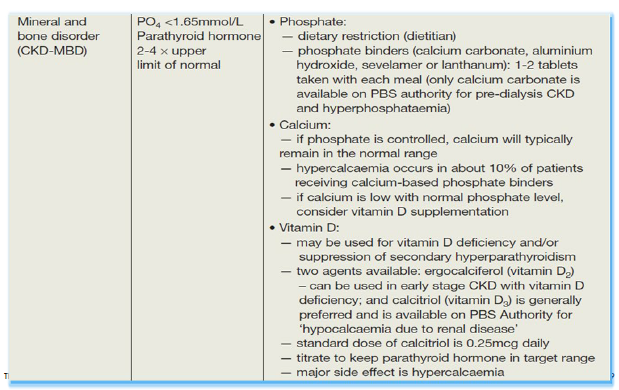
Managing complication - vitamin D and phosphorous metabolism
Kidney intricately involved in bone metabolism
phosphate excretion
vitamin D activation
Abnormalities in these parameters results in
bone pain, increased incidence of bone fractures and deformity, myopathy and muscle pain, and ruptures of tendons
calcification in lungs (impaired pulmonary function, pulmonary fibrosis & hypertension, right ventricular hypertrophy, and cor pulmonale),
heart and coronary arteries (IHD and CHF),
other vascular sites
May be evident with GFR <40mL/min
Goals of treatment
maintain near-normal [Ca 2.2-2.6mmol/L] and [PO4 0.8-1.5mmol/L]
prevent secondary hyperparathyroidism
Management strategies
dietary phosphate restriction
PO4--- binding agents
vitamin D therapy
calcimimetics
dialysis
![<ul><li><p>Kidney intricately involved in bone metabolism</p><ul><li><p>phosphate excretion</p></li><li><p>vitamin D activation</p></li></ul></li><li><p>Abnormalities in these parameters results in</p><ul><li><p>bone pain, increased incidence of bone fractures and deformity, myopathy and muscle pain, and ruptures of tendons</p></li><li><p>calcification in lungs (impaired pulmonary function, pulmonary fibrosis & hypertension, right ventricular hypertrophy, and cor pulmonale),</p></li><li><p>heart and coronary arteries (IHD and CHF),</p></li><li><p>other vascular sites</p></li></ul></li><li><p>May be evident with GFR <40mL/min</p></li><li><p>Goals of treatment</p></li><li><p>maintain near-normal [Ca 2.2-2.6mmol/L] and [PO4 0.8-1.5mmol/L]</p></li><li><p>prevent secondary hyperparathyroidism</p></li><li><p>Management strategies</p></li><li><p>dietary phosphate restriction</p></li><li><p>PO4--- binding agents</p></li><li><p>vitamin D therapy</p></li><li><p>calcimimetics</p></li><li><p>dialysis</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/ac16539f-c6eb-406a-966b-5482e4346f5a.png)
Managing complication - vitamin D and phosphorous metabolism: phosphate and calcium management
Phosphate management:
dietary restriction of protein often reduces PO4 intake
PO4 binding agents
Binds to dietary phosphate to prevent absorption
Calcium containing preparations (e.g. CaCO3)
Sevelamer & Lanthanum available for patients on dialysis
Aluminium hydroxide (not first line due to accumulation and risk of encephalopathy)
Calcium:
If phosphate is controlled, calcium will typically remain in normal range
If level is low with normal phosphate consider vitamin D supplementation
Hypercalcaemia associated with increased risk of vascular calcification
Vitamin D therapy (calcitriol, paricalcitol):
suppresses PTH secretion and stimulates GI calcium absorption
care to avoid hypercalcaemia (less risk with paricalcitol) – monitor levels closely
If kidney function is still intact cholecalciferol can be used
Cholecalciferol may still be used for 25OH Vitamin D deficiency including in combination with calcitriol in advanced CKD
Calcimimetics (cinacalcet)
rapidly suppresses PTH secretion without increasing calcium absorption (risk of hypocalcaemia)
Cinacalcet can be used to treat hyperparathyroidism for individuals on dialysis
PBS delisted from August 2015
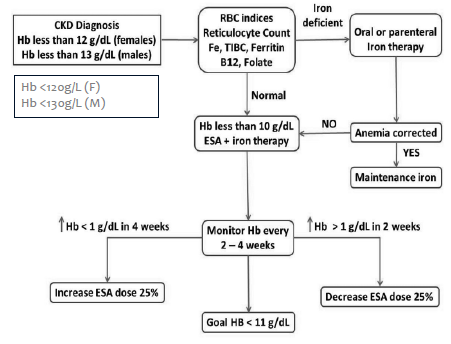
Managing complication - anaemia
Anaemia common in CKD
Due to reduced EPO synthesis
Reduced absorption of iron
Resistance to the action of erythropoietin stimulating agents (ESA)
Targets:
Hb 100-115g/L
Prior to commencing erythropoietin stimulating agents (ESA), trial of iron supplementation is recommended maintaining transferrin saturation (TSAT) >20% and ferritin between >100 μg/L
Once ESA commenced, maintain: Ferritin 200-500 μg/L; TSAT 20-30%
Treatment can decrease morbidity/mortality, reduce LV hypertrophy, increase exercise tolerance, increase QOL
May sometimes respond to Fe infusions, but commonly managed using ESA
Use ESA cautiously- higher Hb levels associated with increased risk of cancer, death, cardiovascular events, and hospitalisation for CHF
Hb should not exceed 130 g/L (increased risk of CV events)
Note: AMH recommends not to exceed 120g/L
TREAT (Trial to reduce cardiovascular events with Aranesp Therapy) failed to show a benefit in outcomes, but treatment with ESAs was associated with increased stroke (NEJM 2009;361)
Most patients using ESA will also require Fe, and potentially folate and/or B12
Ensure transferrin saturation 20%-30% and ferritin between 100 and 500μg/L
4 ESAs currently marketed in Aust:
Darbepoetin alfa (Aranesp)
Epoetin alfa (Eprex)
Epoetin beta (NeoRecormon)
Methoxy polyethylene glycol-epoetin beta (Mircera)
All marketed for treatment of anaemia associated with CKD
All appear to have similar efficacy, most differences related to duration of action, e.g. Eprex dosed twice weekly, Aranesp once weekly and Mircera once monthly
Determining when dialysis is needed
A mnemonic, AEIOU, is a simplified way for determining when dialysis needs to be initiated
Acidosis refractory to medication
Electrolyte abnormality (particularly hyperkalemia)
Intoxicants that are removed through dialysis
Overload (or volume overload) refractory to diuretics
Uremia (which causes the symptoms of kidney failure)
Dialysis - general
Progression of uraemic symptoms and/or hyperkalaemia will result in ESKD patients requiring dialysis or transplant
Two main modalities exist
peritoneal dialysis (PD)
catheter is inserted into abdominal cavity
dialysate is run into abdomen and left for 30 minutes
run into a collecting bag »Multiple times per day
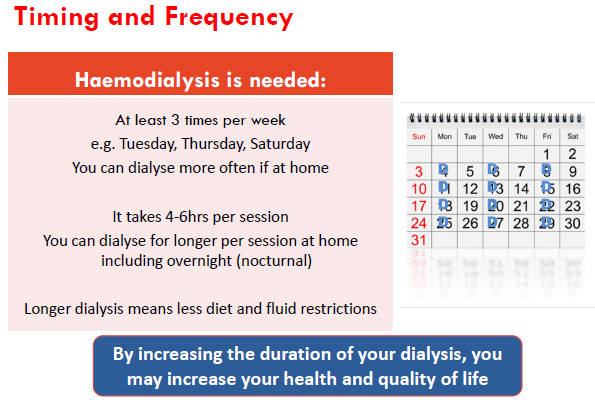
haemodialysis (HD)
blood (heparinised) pumped through dialyser (artificial kidney) and returned to patient.
Multiple times per week for hours
Haemodialysis
In hemodialysis, blood is pumped from the body through a machine where chemistries are balanced and waste and fluid are removed.
The blood is then returned from the machine to the body.
AV fistula:
The preferred access for hemodialysis is an AV fistula.
A surgical connection is made between an artery and vein.
The high pressure and fast flow from the artery traverses through the newly connected vein.
As the access matures, the vein gets larger. It will allow for placing two needles in the access and fast blood flow to facilitate dialysis.
The enlarged vein allows for blood flow rates of > 400 mL / min.
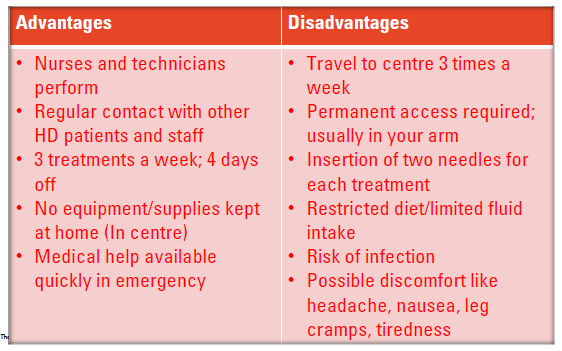
Haemodialysis - advantages and disadvantages
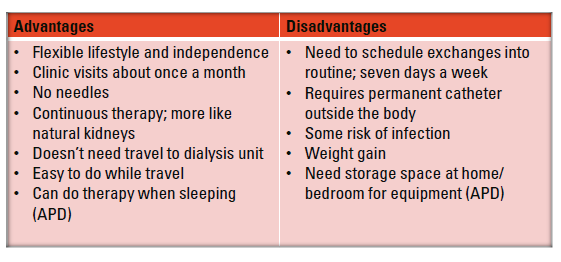
Peritoneal Dialysis (PD)
Peritoneal dialysis can be done in two ways.
Through manual exchanges, known as continuous ambulatory peritoneal dialysis (CAPD).
Overnight, through a cycler, known as automated peritoneal dialysis (APD)
The lining of the peritoneal cavity is covered in small capillaries that act as a natural filter.
In peritoneal dialysis, a catheter is placed in this cavity to allow clean, chemically balanced fluid to be infused.
This fluid helps draw out waste, balance chemistries, and remove excess fluid.
The now dirty fluid is drained and replaced by clean fluid to start the process again.
Peritoneal Dialysis (PD) - advantages and disadvantages