Mass number and Atomic number
0.0(0)
Card Sorting
1/8
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
9 Terms
1
New cards
protons
Positively charged
Relative mass 1
Relative charge +1
2
New cards
Neutrons
Neutrons are electrically neutral
Relative mass 1
Relative charge 0
3
New cards
Electrons
Relative charge -1
Relative Mass 1/2000
Orbit around the nucleus in shells or orbital
4
New cards
Nucleus
Located in center of the atom
Neutral
Small and dense
5
New cards
Mass number ( A )
the total number of nucleons ( protons + neutrons ) in the nucleus
Top number on p table
6
New cards
Atomic number ( z)
The number of proton in the nucleus, identifies the element
Bottom number in p table
Protons = z number
7
New cards
Negative ions
have more electrons than protons
Eg ; A particle with 1 proton and 2 electrons → negative ion charge of -1
8
New cards
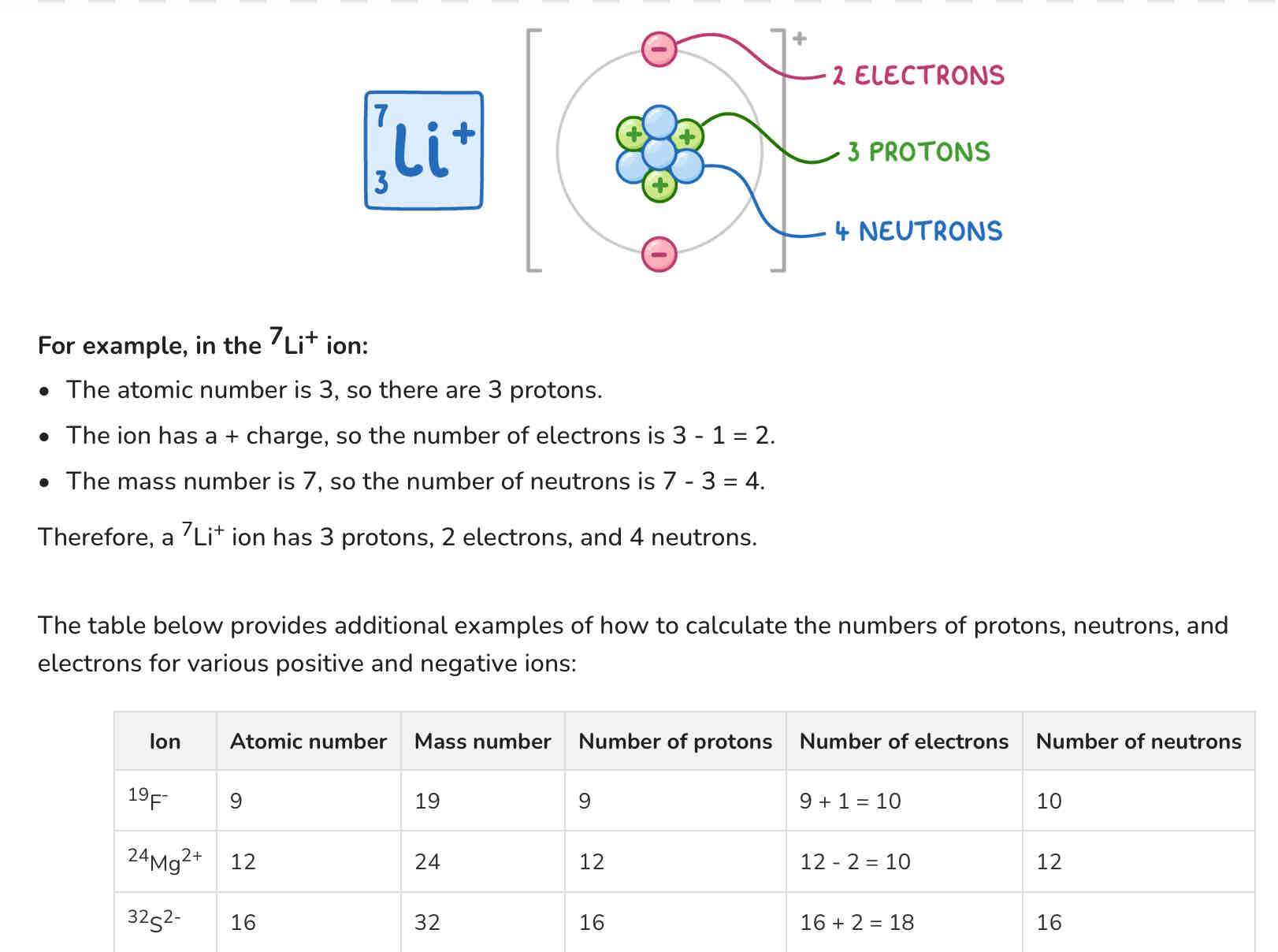
9
New cards