Chem 43A Study Guide
1/153
Earn XP
Description and Tags
Organic Chemistry Lab with Haim Weizman
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
154 Terms
What is the purpose of experiment 1?
Exp #1
Purify an unknown organic solid using recrystallization and identify it based on its melting point by performing both macroscale and microscale recrystallization
Short Protocol of Experiment 1
Exp #1
Macroscale Recrystallization: Dissolve impure solid in hot water → add charcoal → hot gravity filtration w/ pre-warmed funnel → slow cooling → vacuum filtration (Büchner funnel) → dry crystals.
Microscale Recrystallization: Dissolve a small amount of solid in hot water → add charcoal → gravity filtration w/ pre-warmed funnel into Craig tube → slow cooling → centrifugation → dry crystals.
Difference between Macro & Micro -scale recrystallization:
Exp #1
Macroscale is more suitable for purifying larger quantities (0.5-1.0g) and uses larger volumes and simpler methods like suction filtration.
Microscale is more efficient for smaller quantities (10-100mg) and uses more controlled conditions and specialized equipment like centrifugation
How do the results of percent recovery and purity differ between macroscale and microscale recrystallization?
Exp #1
Macro produces more products because the starting masses and volumes are larger. The % recovery should be similar because it's unaffected by starting mass or volume. In practice, micro recrystallization has lower purity, but it can be the same. Purity is dependent on good hot gravity filtration and slower cooling. Micro cools more quickly than macro because there is a smaller amount
Recrystallization definition:
Exp #1
A method to purify a solid by dissolving it in minimal hot solvent and then cooling the solution to form pure crystals, leaving impurities in the liquid
What characteristics affect the recrystallization process?
Exp #1
Polarity of solvent, solvent volume, solubility of compound, rate of cooling, temperature control, uses of seed crystal, and rate of filtration
Why is temperature control important during the recrystallization process?
Exp #1
Too much heat causes overdissolution, leading to loss of products, while insufficient heat could prevent complete dissolution.
Why is selecting the right recrystallization solvent so important?
Exp #1
A good solvent dissolves the compound at high temperatures and allows it to precipitate as the solution cools.
What is the purpose of boiling chips?
Exp #1
Ensure smooth and controlled boiling by creating nucleation sites for small, consistent bubbles to prevent liquid from overheating or erupting.
What is hot gravity filtration, and why is it used?
Exp #1
Used to remove insoluble impurities from the hot solution by using a pre-warmed funnel to avoid premature crystallization
Why do we use charcoal in recrystallization? Why is it critical to prevent charcoal particles from passing into the filtrate?
Exp #1
Remove colored impurities, but if it passes through, it can contaminate the final product by altering the purity and melting point
What is the purpose of a water trap in suction filtration?
Exp #1
Prevent water from flowing backward into the filtration flask and contaminate/destroy the product or equipment
What is the function of centrifugation in microscale recrystallization?
Exp #1
Rapid rotation helps settle the solid crystals at the bottom of the Craig tube for easy seperation from solvent
How do you induce crystallization if it doesn’t occur on its own?
Exp #1
Scratching the glass surface with a spatula, adding a seed crystal, or cooling the solution more slowly
How does cooling affect crystallization?
Exp #1
Slow cooling allows for larger, purer crystals to form as fast cooling tends to trap impurities inside smaller crystals
Melting point definition
Exp #1
The temperature at which a substance transitions from solid to liquid. Used to confirm the identity and assess the purity of the compound
Melting point of pure vs impure substance
Exp #1
Pure substances have a narrow melting point range, and impure substances have a lower and broader melting point range
Why is mixed melting point considered effective?
Exp #1
Confirms if unknown is the same as known. If not the same, the mixed sample is lower and broader. If the same, mixed samples fall within the range of the unknown.
What are the characteristics of a good recrystallization solvent?
Exp #1
Solvent is inexpensive, low toxicity/hazard, doesn't react with compounds or impurities, has a lower boiling point for easy evaporation, dissolves the compound well at high temperatures but poorly at room temperature to allow crystal formation upon cooling, and should either dissolve impurities very well to remain in the solution or very poorly to be filtered out.
Why did we put our crystal in a desiccator overnight?
Exp #1
To keep crystals dry and remove leftover solvent that could affect purity and melting point.
Hazard vs Risk:
Exp #1
Hazard = what can cause harm. Risk = how likely and how bad the harm will be
% Recovery formula
Exp #1
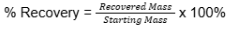
Correction Factor formula
Exp #1
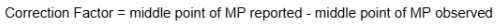
What are the intermolecular forces that form crystals?
Exp #1
London dispersion forces, Dipole-dipole interactions, Ion-Dipole forces, and Hydrogen bonding (dashed lines)
London dispersion forces definition
Exp #1
Weak, temporary attractive forces that occur between all molecules due to fluctuations in electron density
Dipole-dipole interactions definition
Exp #1
Attractive forces that occur between polar molecules due to the attraction between the partial positive end of one molecule and the partially negative end of another
Ion-dipole interactions definition
Exp #1
Attractive forces between an ion (positive/negative) and a polar molecule (partially negative/positive)
Hydrogen bond definition
Exp #1
A hydrogen atom covalently bonded to a highly electronegative atom (N, O, F) is attracted to a lone pair of electrons on another electronegative atom
You determine that compound A and compound B both melt over the range of 134–137 °C. How would you determine if compounds A and B are identical?
Exp #1
-Perform a mixed melting point. If the mp range remains near 134-137 Celsius, then A=B. If the mp range is lower or broader, then A and B are different.
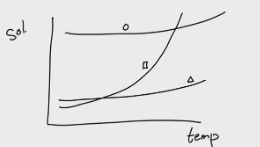
Solubility vs Temperature Graph:
-If we have a mixture of these compounds, add water (solute), and continuously stir. Which compound will dissolve first?
-Now we increase the temperature, which compound will dissolve first?
-If we increase the temperature until the point all the squares dissolve, will the triangle dissolve?
Exp #1
→ The circle will dissolve first because it has high solubility and will dissolve right away
→ Square because it reaches full dissolution at a lower temperature
→ Not so much because it needs more temperature to fully dissolve
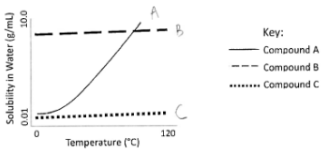
A student conducted a macroscale recrystallization of an unknown that contained a mixture of 3 compounds (A, B, C) and wanted to recrystallize only one of the compounds. Use the graph to help you answer the following questions.
The student dissolved the sample in boiling water and noticed that some of the mixture did NOT dissolve into the water. They decide to hot gravity filter this solid. Using the solubility graph above, which compound(s) will not have dissolved in the water? (Circle all that apply)
Compound A Compound B Compound C Cannot be determined
The student collected the liquid that went through the filter during the hot gravity filter and then cooled down the solution to recrystallize a product. Which of the following compound(s) will stay in solution and not recrystallize? (Circle all that apply)
Compound A Compound B Compound C Cannot be determined
Answer:
-Compound C because barely soluble at high temp
-Compound B because solubility doesn’t change with temp.
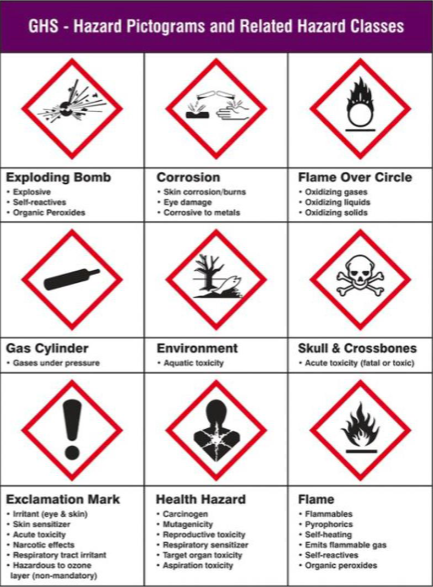
What are the different types of the Globally Harmonized System (GHS) pictogram?
Exp #1
Explosive, Corrosive, Oxidizer, Compressed Gas, Environment Hazard, Acute Toxicity, Harmful, Health Hazard, and Flammable
What is KD (distribution coefficient)?
Exp #2
The ratio of a compound’s solubility in two immiscible solvents - organic and aqueous. Determines how a compound will partition itself between the two solvents during extraction.
KD =[solute in organic layer]/[solute in aqueous layer]
IfKD>1, the solute prefers the organic layer.
If KD<1, the solute prefers the aqueous layer.
KD = 5 means the concentration of the compound in the organic layer is 5 times higher than in the aqueous layer.
What is the purpose of experiment 2?
Exp #2
Part 1: Determine the Kd of benzoic acid between MTBE and H2O.
Part 2: Isolate caffeine from tea leaves by using liquid-liquid extraction and purify it by sublimation
Short Protocol of Experiment 2:
Exp #2
Part 1: Mix benzoic acid, water, and MTBE → transfer aqueous phase → dry with magnesium sulfate → filtering pipette with cotton → Evaporate and calculate Kd
Part 2: 2 Solid-Liquid Extraction (boiling the tea) → 2 Liquid-Liquid Extraction (add ethyl acetate and transfer organic phase) → Dry with magnesium sulfate → filtering pipette with cotton → Evaporation of solvent (hotplate) → Sublimation by placing icebath on top with hotplate
What group does caffeine belong to, and why is that group special?
Exp #2
Belongs to alkaloids and they contain nitrogen atoms and have basic properties that allow them to form salt with acids.
Solid-liquid extraction definition:
Exp #2
Solid material (tea leaves) is mixed with a solvent to dissolve specific compounds from the solid into the liquid phase based on their solubility
Liquid-liquid extraction definition:
Exp #2
Distributing a compound between two immiscible solvents based on solubility, then separating the layers to isolate the desired compound.
Why is ethyl acetate used for liquid-liquid extraction?
Exp #2
Ethyl acetate is a polar, non-protic solvent, making it effective in extracting caffeine from water by taking advantage of caffeine's higher solubility
In a liquid-liquid extraction, which solvent will form the upper layer or the bottom layer? What is a quick and simple technique to identify either layer?
Exp #2
The upper layer is the organic solvent (MTBE or ethyl acetate) because it is less dense. The bottom layer is the aqueous solvent (water) because it is more dense. By adding a small drop of solvent to see which layer it resides with.
Sublimation definition:
Exp #2
A substance transitions from the solid to the gas phase without passing through the liquid phase. The gas then condenses back into solid form upon cooling.
Why Use Sublimation in Caffeine Purification?
Exp #2
Takes advantage of caffeine's ability to transition from a solid to a gas, leaving impurities that don't sublime behind and allow pure caffeine to condense back into a solid form.
What are the intermolecular forces involved in caffeine extraction?
Exp #2
Caffeine, a polar compound containing nitrogen atoms, interacts with solvents through dipole-dipole interactions and hydrogen bonding, allowing it to dissolve in both aqueous and organic solvents
Solubility definition
Exp #2
The ability of a substance to dissolve in a solvent at a given temperature or pressure
Why do we use magnesium/sodium sulfate? How do you know if the magnesium sulfate has worked?
Exp #2
It is a drying agent that removes traces of water from the organic phase. It works when the drying agents are not clumped together and are free to form a “snow globe” effect, otherwise add more.
What is the chemistry behind how and why caffeine affects us?
Exp #2
Caffeine blocks adenosine receptors in the brain that promote sleepiness by binding to those receptors. Additionally, caffeine prevent the breakdown of the enzyme cAMP-PD, which increase blood pressure and oxygen to the brain to prolong alertness effects.
Why do we put cotton down a Hirsch funnel?
Exp #2
To filter unwanted solid impurities (like magnesium sulfate) from the organic phase to ensure the liquid phase is free of particles before evaporation.
Why do we place an ice bath on top of the petri dish in part C sublimation?
Exp #2
Creates a cold surface on the petri dish’s lid to condense the sublimed caffeine vapor to crystallize back into the solid form.
What would happen in the extraction if you forgot to add Na2CO3 (sodium carbonate) during the brewing of the tea? Briefly discuss both the chemistry and how this would affect the process and results.
Exp #2
The pH remains low, so caffeine stays in its protonated form in the aqueous layer, tannins are not hydrolyzed and remain uncleaved, causing them to partition into the organic ethyl acetate layer and contaminate the final extract.
Due to its moderately safe nature, we will use ethyl acetate for the caffeine extraction. (Note: Ethyl acetate, MW = 88.1, density = 0.90 g/mL, bp = 77.1°C). If a liquid-liquid extraction was conducted using 3 mL of brewed tea and 3 mL of ethyl acetate, and assuming a K₀ of 3:
a. Which layer would more of the caffeine reside in – organic or aqueous? Why?
b. Is the caffeine found in the top or the bottom layer? Why?
c. What percentage of caffeine is recovered after one extraction?
Exp #2
a. A Kd > 1 tells us that caffeine is more soluble (resides) in organic than in the aqueous phase.
b. Caffeine is in the top layer because ethyl acetate has a lower density than water.
c. Kd = organic/aqueous = 3/1 → 3/3+1 = ¾ x100% = 75%
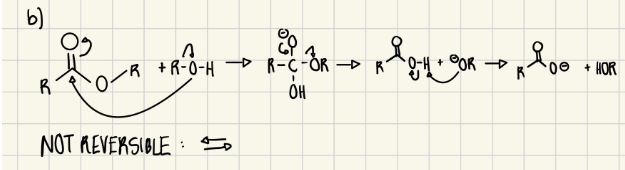
The procedure calls for a weak base, Na₂CO₃, to be present during the initial extraction of caffeine from the tea leaves into hot water.
a. Give one reason why the procedure calls for a weak base.
b. Provide a mechanism for the reaction with the weak base. (Exact chemical structures are not required; you may use chemical shorthand, ex., R–OH.)
Exp #2
a. To base hydrolysis ester bonds to break up tannin into smaller water-soluble molecules. Deprotonates caffeine so it's non-ionic and more soluble in the non-polar organic phase.
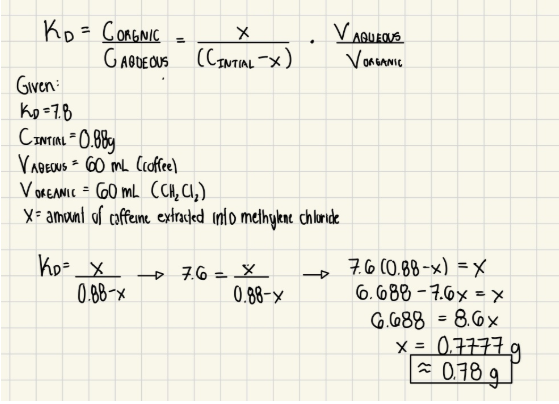
Sequential Extractions Example:
Imagine that the world’s most potent coffee contains an amazing 0.88g of caffeine in a 60 mL serving. The Kd for caffeine is 7.6 for methylene chloride and water. If the super coffee is extracted with 60 mL of methylene chloride (and we assume that all other ingredients are 100% water-soluble), how much caffeine is removed from the water? Show your calculation.
Exp #2
Sequential Extractions Example:
Assume starting mass is 0.88g and Kd = 7.6 still. Alternatively, the 60 mL of coffee solution is extracted with 3 portions of 20 mL of methylene chloride. How much caffeine is left in the water after the extractions? Show calculations for each step of the three extractions AND using a quick formula.
Exp #2

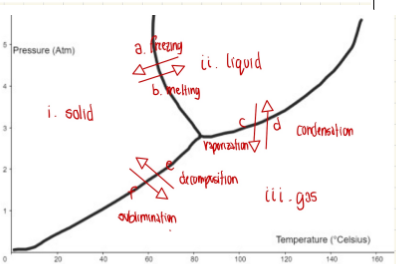
Chart of transition phase
Exp #2
What is the purpose of experiment 3?
Exp #3
To separate and identify an amine, a carboxylic acid, and a neutral organic compound from an unknown mixture using acid-base liquid-liquid extraction and melting point analysis.
Short Protocol of Experiment 3
Exp #3
-Dissolve the unknown solid in MTBE to create the mixture.
-Amine Extraction: Extract 3 times with 5% HCl, transfer the aqueous layer, wash with fresh MTBE, basify with NaOH, suction filtration, and dry crystals in a desiccator.
-Carboxylic Acid Extraction: Extract 3 times with 5% NaOH, transfer the aqueous layer, wash with MTBE, acidify with HCl, suction filtration, and dry crystals in a desiccator.
-Neutral Compound Extraction: Extract with H2O, remove water, add magnesium sulfate, filter pipette with cotton, and evaporate in the fume hood and desiccator.
Why do we add 5% HCl during the extraction of the amine, 5% NaOH during the extraction of carboxylic acids, and water for the neutral?
Exp #3
5% HCl protonates the amine and 5% NaOH deprotonates carboxylic acids to make them water-soluble; water separates neutral compounds into the organic layer.
What happens if we didn’t add HCl to protonate the amine or NaOH to deprotonate the carboxylic acid?
Exp #3
The amine would stay unprotonated, and the carboxylic acid would stay protonated, causing both to stay in the organic layer, preventing their separation and isolation.
Why do we wash the aqueous layer with MTBE and transfer it to a new tube?
Exp #3
MTBE is a non-polar, immiscible solvent used to extract remaining organic compounds from the aqueous layer and reduce contamination.
What should you do if the precipitate didn’t form for amine and carboxylic acid?
Exp #3
Extract aqueous layer with 3×3 mL MTBE, dry with MgSO₄, transfer to tared vial, and evaporate in hood.
What would happen if we used a stronger acid for the amine extraction or a stronger base for the carboxylic acid extraction?
Exp #3
Other necessary basic/acidic compounds would also be protonated/deprotonated and this would prevent selective extraction and loss of purity,
Why did we evaporate our neutral in the fume hood but not the amine or carboxylic acid?
Exp #3
The neutral is more volatile and has a lower melting point, which could release hazardous vapors, while amine and carboxylic acid will not melt at room temperature because it less volatile and more stable.
Potential errors that can affect the recovery of our unknown mixture.
Exp #3
Incomplete layer separation, misidentifying layers, incorrect acid-base amounts, leftover MTBE, or filtration errors.
Polar vs nonpolar molecules
Exp #3
-Polar molecules have an uneven distribution of electron density and different electronegativity, resulting in partial positive and negative ends (dipole)
-Non-polar molecules have an even distribution of electron density due to symmetrical geometry and similar electronegativities
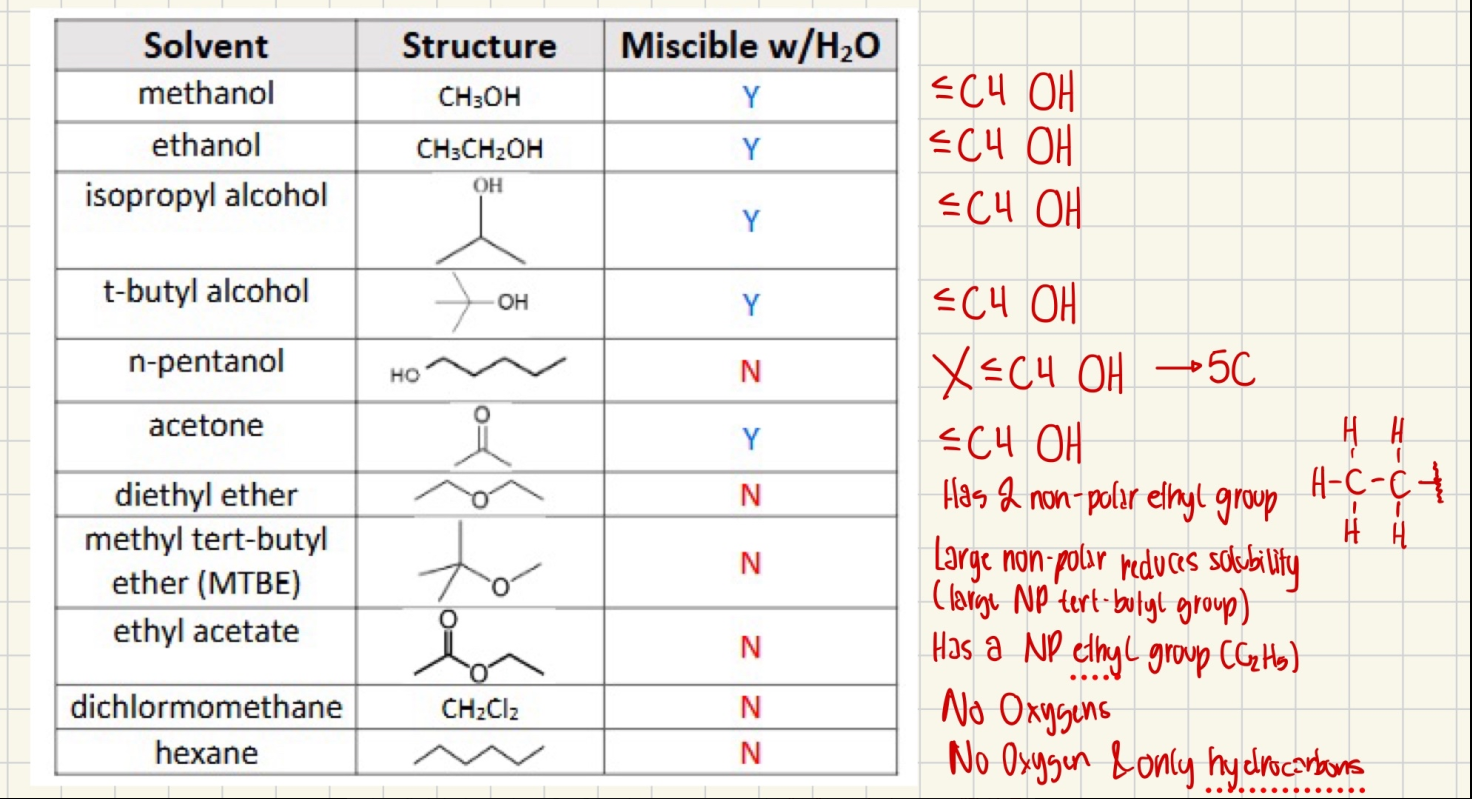
When is a compound water-soluble?
Exp #3
More soluble if it is polar, can form hydrogen bonds, and has ionic charges with smaller compounds (≤ C4) like carboxylic acids, amines, and alcohols. Less soluble for larger nonpolar molecules like hydrocarbons or alkyl halides, which are limited by hydrogen bonds.
Why do we perform multiple extractions with smaller amounts (3x3 mL) instead of a single extraction?
Exp #3
Multiple extractions reduce the concentration gradient between organic and aqueous, allowing more compounds to transfer into the aqueous phase and increasing the yield
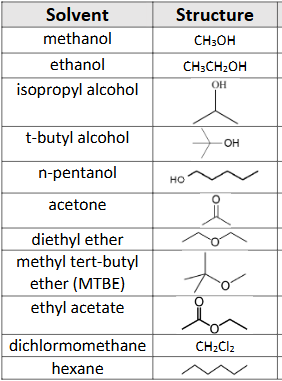
What solvents are miscible with water?
Exp #3
Why does asking which compounds would be soluble in an aqueous solution that is acidic (pH <7) versus basic (pH > 7) make a difference?
Exp #3
pH < 7, acids are neutral (less soluble), and bases are protonated (soluble in water).
pH > 7, acids deprotonate (soluble in water), and bases are neutral (less soluble).
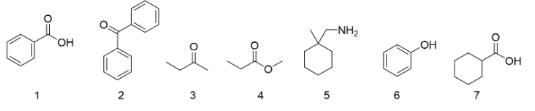
Which of these compounds would be soluble in an aqueous solution of pH=11?
Exp #3
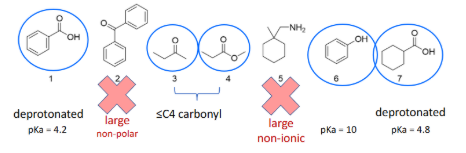
Since ions and salts greatly improve water solubility, how do you determine if a functional group (F.G.) will be an ion?
Exp #3
Use the Henderson-Hasselbalch equation to compare pH and pka values. pH > pKa, the functional group is deprotonated and exists as an ion. pH < pKa, the functional group will likely remain protonated and be neutral
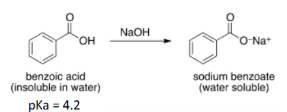
At what pH will Benzoic Acid be roughly split equally between water and MTBE? Fully in MTBE?
Exp #3
-Split equally when there is 50% dissociation between the conjugate base and acid. → pH = 4.2
-Full dissociation when pH=pKa +2, there is mostly conjugate base → pH = 4.2 + 2.0 = 6.2
-full protonation when pH=pKa – 2, there is mostly acid → pH = 4.2 - 2.0 = 2.2
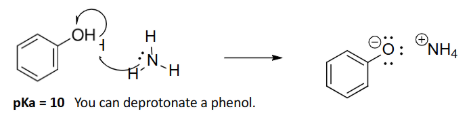
Isn’t the aromatic ring too non-polar to be water-soluble?
Exp #3
@pH = 10, it is 50% protonated. @pH = 11, it is partially deprotonated and soluble in water
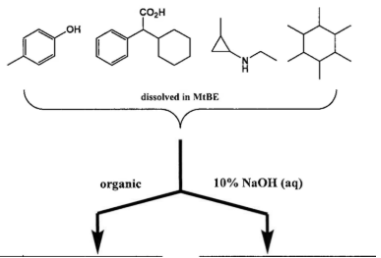
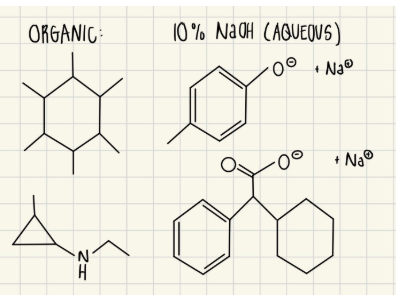
You have a mixture of the following four compounds dissolved in MTBE and perform a liquid-liquid extraction to separate them. Complete the following extraction/separation scheme by drawing the exact structures of the compound(s) that are present in the respective aqueous/organic layers in the boxes provided.
Exp #3
Adding NaOH (strong base) deprotonates acidic groups like hydroxyl (OH) and carboxylic acid (COOH) and moves them to the aqueous phase
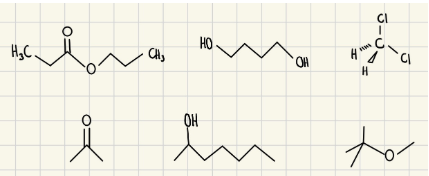
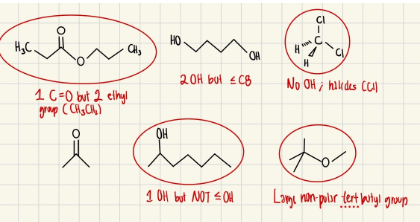
Circle the organic liquids that are immiscible with H2O
Exp #3
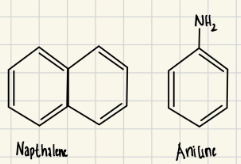
Which compound would you be able to remove using an aqueous extraction? What would you add to the aqueous phase to help extract this compound from the dichloromethane? Explain how this process works.
Exp #3
The aniline could be removed using an aqueous extraction by adding acid to form a charged amine that is soluble in the aqueous layer, while naphthalene is nonpolar and remains in the organic layer
How would you isolate amine from the aqueous phase in part A? How would you isolate the solid from the other compound? (The compound was not isolated in part B but the Neutral)
Exp #3
→ Neutralize the aqueous solution by adding NaOH (Base) and filter out the solid precipitate and dry
→ Use magnesium sulfate to remove water from the organic layer and dry in a fume hood.
Simple Flowchart of Separation of Mixture in Exp 3
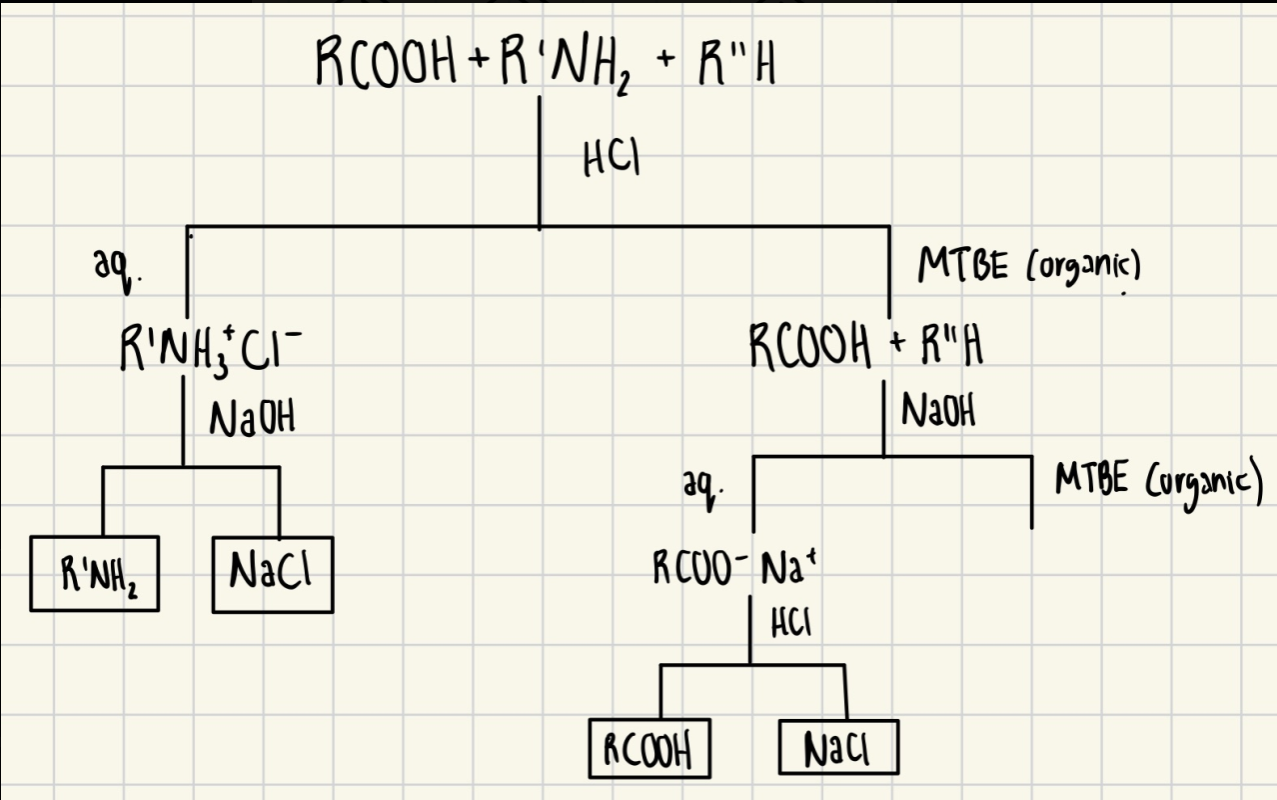
What is the purpose of experiment 4?
Exp 4
Use thin-layer chromatography (TLC) for analysis and column chromatography for purification to separate and identify components based on differences in polarity and interactions between the stationary and mobile phases
Short Protocol of Experiment 4
Exp 4
-Part A Part I: TLC of ortho- and para-Hydroxyacetophenone (Hydroxy-as-toop-pho-none) co-spot in 30:70 ethyl acetate:petroleum ether
-Part A: Part II: TLC of unknown and known standards (acetylsalicylic (acetyl-sal-cylic) acid, acetaminophen (uh-see-tuh-mi-nuh-fin), caffeine, ibuprofen) in 8:2 ethyl acetate:petroleum ether
-Part B: Column chromatography of a mixture containing ferrocene, acetylferrocene, and 9-fluorenone is eluted with increasing polarity solvents to collect fractions, analyzed on TLC, evaporated, and the melting points.
What are the 3 factors that determine the distance a compound travels along a TLC plate?
Exp 4
Polarity of compound (solute), polarity of solvent (mobile phase), and polarity of adsorbent (stationary phase)
Stationary vs mobile phase
Exp 4
-The stationary phase is the immobile, solid surface that the compounds interact with during separation.
-The mobile phase is the solvent (or solvent mixture) that moves through the stationary phase, carrying compounds with it.
How does polarity affect the movement of compounds during TLC?
Exp 4
-High polarity compound = stronger interaction with stationary = moves slowly (lower Rf)
-Low polarity compound = weaker interaction with stationary = moves faster (higher Rf)
Why is silica gel used as the stationary phase in this experiment?
Exp 4
It’s a polar, porous material with hydroxyl groups that strongly adsorb polar compounds, enabling separation by polarity.
Why does the solvent rise on the TLC plate?
Exp 4
Due to capillary action through the porous silica network.
Why must you use a pencil, not a pen, to mark TLC plates?
Exp 4
Ink contains compounds that run on the plate and interfere with results.
How does solvent polarity affect Rf values in TLC?
Exp 4
More polar solvents reduce solute binding = higher Rf
less polar solvents increase solute binding = lower Rf.
Rf value formula
Exp 4
Distance traveled by Compound / Distance traveled by Solvent
Why is it important not to let the solvent reach the top of the TLC plate?
Exp 4
The TLC plate will evaporate unevenly, causing spots to move upward and smear, making Rf inaccurate
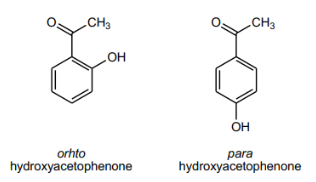
What did you observe on the TLC for the ortho and para isomers of hydroxyacetophenone and why?
Exp 4
Ortho appears higher because it is less polar and para appears lower because it is more polar
Why do ortho and para isomers of hydroxyacetophenone have significantly different Rf values and melting points, even though both compounds have the same functional groups?
Exp 4
Ortho’s adjacent functional group can form intramolecular hydrogen bonds, reducing interactions with silica gel (higher Rf) and has a lower melting point (4–6 °C). Para’s opposite functional group cannot form intramolecular H-bonds, so it interacts more with the silica gel (lower Rf) and has a higher melting point (109–111 °C).
What did we separate in column chromatography? And why that order?
Exp 4
From least polar to most polar: orange ferrocene → 9-Fluorenone → acetylferrocene
Used nonpolar to moderate to polar solvent: petroleum ether, MTBE/petroleum ether, pure MTBE
Order of the layers added in column chromatography (bottom to top)
Exp 4
Cotton plug, sand, silica gel, unknown/silica gel mix, and sand.
Why do we add sand as a layer in our column chromatography?
Exp 4
Create a flat surface for leveling and prevent silica gel from clogging.
In column chromatography, what happens if you add a new solvent too early or let the column run dry?
Exp 4
Adding polar solvent too early can cause premature elution, while letting the column dry introduces air pockets that disrupt separation.
Why is consistent air pressure important during column chromatography?
Exp 4
Inconsistency disrupts solvent flow, causing uneven separation or mixing of fractions.
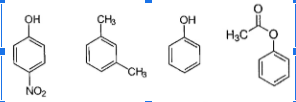
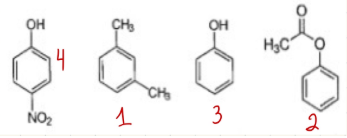
Rank these compounds according to which would elute earliest from a column packed with silica gel and using 1:1 MTBE:hexane as the mobile phase. (1 = first → 4 = last.)
Exp 4
-m-xylene is first because least polar with no oxygen
-phenylacetate next, because it has an ester group (less polar)
-phenol next because of OH (polar)
-p-nitrophenol is most polar because OH and amine (N)
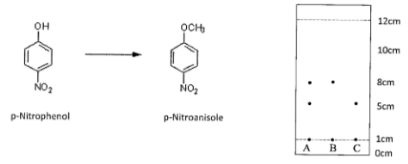
You use thin-layer chromatography to analyze a reaction that has been happening for 1 hour. You remove a small sample of the reaction mixture and spot a TLC plate, along with standards of the starting reagent and the desired product. After development of the plate, you are able to visualize the spots on the plate as shown below. Given that spot A is the reaction mixture, answer the following questions:
A.) If the stationary phase is silica gel, and the eluting solvent is Ethyl Acetate, which spot on the plate (A, B, or C) is most likely the starting material? Support your conclusion.
B.) Calculate the retention factor (Rf) of the starting material. Show your work.
(Fractions are acceptable if you don’t have a calculator.)
C.) Given that the reaction mixture lane shows two spots, do you think that the reaction is complete? Yes or no.
Exp 4
Answer:
A) C because it is more polar due to the -OH group, so it will interact more with the silica so lower Rf
B) Rf = Distance travelled by Compound / Distance travelled by Solvent = (5-1 cm/12-1 cm) = 4/11 cm
C) Lane C = starting material. Lane B = final product. A complete reaction would only show the product spot in lane A
What does it mean when the spots streak or become long ovals on TLC?
Exp 4
The spots ared overloaded, meaning there is more of compound X than bonding sites on the stationary phase.
Decreasing polarity of solvents:
Exp 4
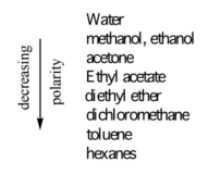
Decreasing polarity of certain functional groups
Exp 4
