Investigating the decomposition of Group 2 carbonates
0.0(0)
0.0(0)
Card Sorting
1/3
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
4 Terms
1
New cards
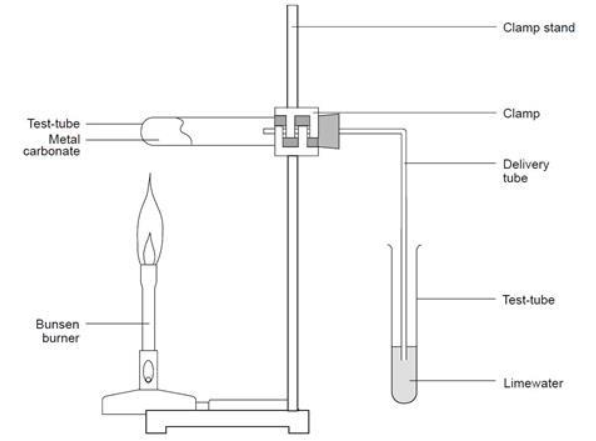
Outline the experiment
Group 2 carbonates become more thermally stable down the group - harder to break down as you go down the group
2
New cards
Explain the procedure
Add Ca(OH)2 (limewater) into test tube - fill with distilled water and stir
filter the Ca(OH)2 and pour solution into conical flask
Add magnesium carbonate to a second test tube
clamp stand - place test tube vertically and tighter
add delivery bung through lime water and attach to magnesium carbonate
heat the magnesium carbonate using Bunsen burner
time how long it takes for lime water to go cloudy
3
New cards
Set Up
4
New cards
.
.