Pceutics Final Exam
1/131
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
132 Terms
granules
aggregates of powder particles formed by the granulation process
can contain one or more APIs with or without other ingredients
commonly used in making tablets or filled into capsules and binding agents
can be swallowed orally, dispersed in food, or dissolved in water
offer additional advantages over powders
larger
size of a granule is __ than powder
less
the surface area of granules is ___ than powder
less
the cohesive forces of granules is __ than the cohesive forces of powders
greater
the flowability of granules is __ than powders because thee are less cohesive forces
greater
stability to the effects of atmospheric humidity is __ for granules than powders because the surface area is less than that of a powder
less
caking or hardening upon standing is __ for granules than powders because the surface area is less than that of a powder and less cohesive forces
better
wettability by a liquid is __ for granules than powders because of larger particle size and reduced surface area and less cohesive forces
wet method
A technique in pharmaceutical formulation that involves dissolving a certain amount of solvent to aid in granulation, leading to improved particle cohesion and flow properties.
moisten the powder and add binding agents to facilitate particle aggregation (liquid bridging) → sieve → dry
dry method
A granulation technique that utilizes no liquid but compacts powders using mechanical forces, often resulting in increased particle size and improved flow characteristics.
powder or powder mixture (binding agents are incorporated into the powder formulation) → compress at high temperature → large tablets → granulate by mills → granules
powder
starting material for both wet and dry methods of preparation of granules
binding agents
what is added to promote aggregation in both wet and dry method of granulation?
binds the particles together (liquid briding)
what purpose does the liquid serve in the wet method?
granules
what type of solid passes through the sieve in the wet method?
wet powder
what type of solid is trapped in the sieve and moved to the oven for drying?
tablets
what type of solid is formed prior to granulation in the dry method?
grinding
what particle size reduction technique is used to produce granules in the dry method
wet method vs dry method
The wet method involves using a liquid binder to agglomerate powders, while the dry method uses mechanical forces to form granules without liquids.
compressed tablets
prepared with punches and ides under very high pressure
compaction of powder/granules
most tablets are these
use automatic tabletting press
molded tablets
prepared by forcing dampened powder material into a mold
moist tablets allowed to dry
soft, soluble and rapidly dissolve
much lower pressure involved
molded tablets
which type of tablet manufacturing requires water?
4 stages of compressed tablet manufacturing
filling→ powder added to hopper in order to get into machine
metering→ removes excess powder, set exact amount per tablet
compression→ high pressure to compress powder to create tablet
ejection→ takes tablet out of the machine
lactose
a common excipient used as a filler in tablet formulations, providing bulk and stability.
sucrose
A type of sugar used as a sweetener in various pharmaceutical formulations and medicines.
dye
a substance used to add color to pharmaceuticals or other formulations, enhancing their appearance and characteristics.
multi layer tablet
A pharmaceutical tablet designed with multiple layers, each potentially containing different active ingredients or excipients, allowing for controlled release or distinct functional properties.
tablet coating
serves several possible purposes
provides strength to the tablet (reduces friability)
masks unpleasant tastes or odors of the tablet
protects the drug from light, moisture, and oxidation
enteric coating prevents drugs from releasing in the stomach
coating can modify drug release (dissolution) form the tablet
separates incompatible materials by selective coating (multi-layered/coated tablets)
can be used for product identity or brand recognition
reduces operator exposure to active substances
tablet coating
include sugar, film, enteric, and controlled release
primary purpose of enteric coating
to prevent drug release in the stomach, allowing for targeted release in the intestine.
primary purpose of controlled release coating
to allow for a gradual release of the drug over an extended period, enhancing therapeutic effect and minimizing side effects.
primary purpose of sugar coating
to improve taste and mask bitterness, as well as to enhance appearance and protect the tablet.
sugar coating
often involves multiple coats adding 30-50% extra mass to the tablet
the process requires 6 steps as depicted
seal tablet core
application of specialized polymer based coating directly to the tablet core
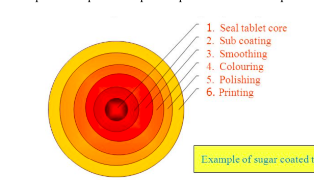
sub coating
provides the rapid buildup necessary to round up the tablet edge

smoothing
even out the tablet surface and fill the irregularities generated during subcoating
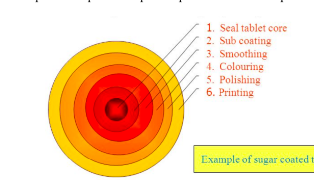
coloring
multiple application of syrup solutions containing the requisite materials necessary to achieve the desired shade
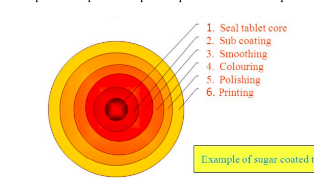
polishing
reduces the very dull appearance and fives them the high degree of gloss that typifies finished sugar coated tablets
accomplished by applying mixtures of waxes
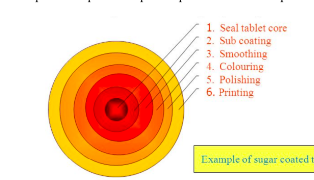
printing
application of special edible inks to the coated tablet surface for identification
film coating
often entails applying a single, thin (20-100 um) polymer coat adding less than 2-3% extra mass to the tablet
difference between film coating and sugar coating
Film coating generally uses a polymer-based layer that adds minimal weight and provides a smooth finish, while sugar coating involves multiple layers of sugar and can significantly increase the tablet's size and weight.
cracking
tablets core expands when exposed to typical coating process temperatures
too dry, too cold

roughness
coating formulation is too viscous
excessive pigments concentration
sprayed droplets dry too rapidly
spraying nozzle is too far
chipping
high degree of attrition associated with the coating process
low amount of polymers
high pan speed

sticking and picking
inefficient drying (low temperature)

peeling
tablet core ingredients do not promote good coating adhesion
adhesive failure
insufficient film strength
cohesive failure

twinning
inefficient drying
shape of tablets is not appropriate (happens to flat surfaces)
too slow pan speed

logo bridging
logo design is inadequate for a film coated product
too high coating viscosity

oral, vaginal, buccal, sublingual
how are tablets administer?
advantages of tablets
dose accuracy, compactness, portability, ease of administration, systemic effects, convenient, stability, preparation, modified release
disadvantages of tablets
variability in absorption process, delayed action, adverse reaction, not long lasting, difficult to compress into tablet, API needs good water solubility, API must be a solid, API must be a small molecule
capsule
solid dosage form containing at least one API and/or insert substances enclosed in a small hard or soft shell
capsule shell
normally made from gelatin and cellulose polymers
hard capsules
consist of two prefabricated cyclindrical sections, body and cap
usually contain powders and granules (but can contain paste, liquid, tablets)
come in a variety of sizes
soft capsules (soft gel)
consist of a continuous gelatin shell (one piece)
contain a semi-solid or liquid fill
come in a variety of shapes
shells are softened by addition of glycerol or sorbitol
2 major types of capsules
hard and soft capsules
powder and granules
what is a hard capsule filled with?
semi-solid or liquid
what is a soft capsule filled with?
easier to swallow
how does the addition of glycerol or sorbitol assist with administration of the dosage form?
gelatin or cellulose polymers
what substances are used to prepare the shells?
collagen is heated to denature and then cooled
how is gelatin produced from collagen?
amino acids
collagen and gelatin are both polymers. what are the monomers that form them called?
liquid
when gelatin cools following denaturation of collagen, it forms a gel that can harden into a shell upon drying. if gelatin is heated, why physical state results?
store in cool area
what are the implication for storage of capsules?
dry
gelatin is stable when ___
nontoxic
since gelatin is edible, it must be ____
soluble
gelatin is ___ in biological fluids at body temperature
HPMC (hydroxypropylmethylcellulose)
derived from plants (bark of pine and spruce trees)
can be used to produce capsules
good for patients who are allergic to gelatin, vegan, or vegetarian
preparation of hard capsules
3 step process
capsule sizes vary in length and volume
step 1: develop and prepare formulation, select the capsule size
step 2: fill the capsule shells, seal the capsule
step 3: clean and polish the filled capsule
soft gel capsule preparation
prepared in a single step process (formed, filled, and sealed) using two common methods
rotary (punching) method, seamless (dropping) method
inversely proportional
the relationship between hard capsule volume and the size number
rotary (punching) method
A technique used in soft gel capsule preparation where the capsules are formed and filled simultaneously by pressing a die to create individual capsules.
two film sheets are placed between two die rolls while injecting a liquid such as medicine between the two sheets, and the dies perform pressured formation, sealing and cutting of the capsules
seamless (dripping) method
A soft gel capsule preparation technique where capsules are formed without seams by dropping a liquid formulation into a mold. This method allows for a continuous production process.
a liquid such as medicine is dropped from the inner section of a double concentric nozzle while film liquid containing a gelling agent form the outer section, and round seamless capsules are formualted by means of the interfacial tension
advantages of capsules
dose accuracy, compactness, portability, ease of administration, mask unpleasant taste, convenient, non-water soluble liquids (fish oil), modified release
disadvantages of capsules
variability in absorption process, delayed action, adverse reactions, not long lasting, manufacturing, not suitable for hygroscopic or deliquescent drugs (absorb water from the shell), not suitable for efflorescent drugs (soften the capsules)
release
rate limiting step of controlled release drug systems
drug in dosage form→ drug at absorption site
absorption
rate limiting step of the immediate release dosage forms
drug at the absorption site→ drug in the body
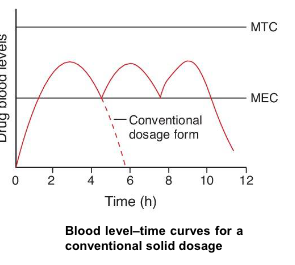
conventional (immediate-release) dosage forms
when they are taken on schedule and more than once daily, they cause sequential therapeutic blood level peaks and valleys (troughs)
however, when doses are not administered on schedule, the resulting peaks and valleys reflect less than optimum drug therapy
if doses are administered frequently, minimum toxic concentrations of drug may be reached, with toxic side effects resulting
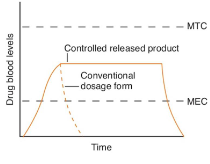
modified release (MR) dosage forms
refer to the modification of drug release from a dosage form with the specific aim of delivering drugs at
desired rates
specific sites in the gastrointestinal tract
most are orally administered tablets and capsules
other forms include ocular, parenteral, sub-dermal, and vaginal products, transdermal patches
classification: delayed release, extended release
many terms such as sustained release, sustained action, prolonged action, controlled release, extended release, timed release, and long action have been used interchangeably
on schedule and more than once daily
when immediate release dosage forms are taken ____ they cause sequential therapeutic blood level peaks and valleys (troughs)
less than optimum drug therapy
when immediate release dosage forms when doses are not administered on schedule, the results peaks and valleys reflect _____
toxic side effects resulting
if immediate release dosage forms are administered too frequently, minimum toxic concentrations of drug may be reached with _____
classification of modified release dosage forms
delayed release
extended release
delayed release
release the drug at a time or location later than immediately after administration
extended release
also called prolonged release or sustained release
drug plasma levels are sustained for longer periods of time
gastro-retentive systems
extended release systems which are retained in the stomach
floating on the surface of gastric fluid or attaching to the mucus surface of the stomach
sustained release
SR
sustained action
SA
prolonged action
PA
controlled release
CR
extended release
ER
timed release
TR
long acting
LA
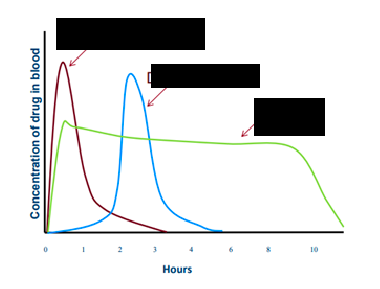
immediate release
red line
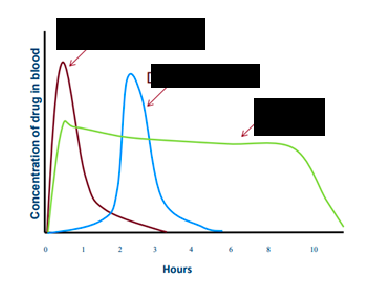
delayed release
blue line
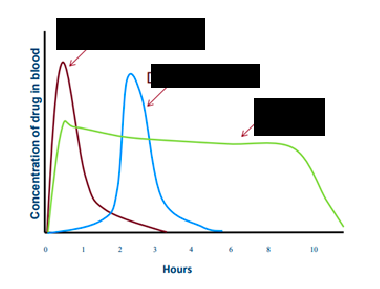
extended release
green line
advantages of modified release dosage forms
less fluctuation in drug blood levels
less frequent administration
enhanced convenience and compliance
reduction in adverse side effects
reduction in overall health care costs
disadvantages of modified release dosage forms
loss of flexibility in adjusting the drug dose and/or dosage regimen (you can’t split the dose or take 2 doses at once)
dose dumping: sudden and total drug release
dose dumping
the rapid release of the entire dose of a drug from a modified release formulation, potentially leading to toxicity or adverse effects.
causes of dose dumping
improper coating
the dosage form is chewed or crushed prior to ingestion
prevention of dose dumping
sufficient coating should be applied uniformly across the surface of the material that is to be coated
educate patients (the dosage form should not be chewed or crushed prior to ingestion)