KMT, Matter & Gas Laws Test
1/64
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
65 Terms
What is matter?
Anything that has mass and takes up space (has volume)
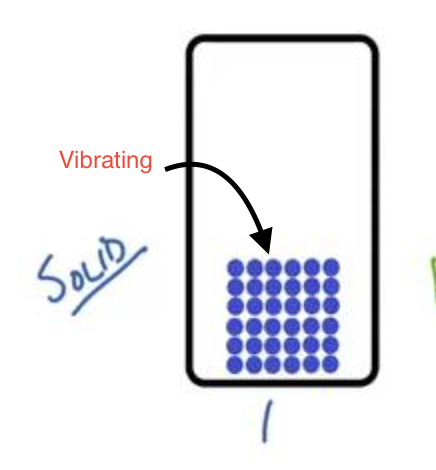
What is a solid? (volume, shape, flow, compressible, KE, IM forces)
Matter that has a definite shape (cannot flow), as particles are too close, a definite volume, particles are tightly packed, and it is not compressible. Has vibrational energy, low kinetic energy, and strong intramolecular forces.
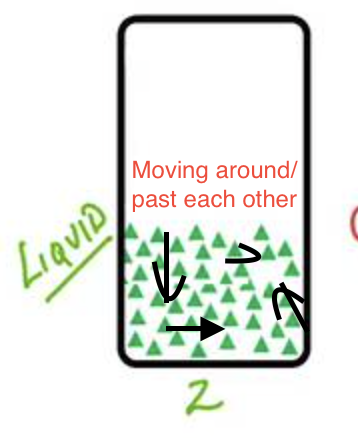
What is a liquid? (volume, shape, flow, compressible, KE, IM forces)
Matter that has a definite volume, takes the shape of its container, particles move past each other (flows), particles are less tightly packed, and it is not really compressible. Particles often interact with more kinetic energy than a solid, but still have strong intramolecular forces.
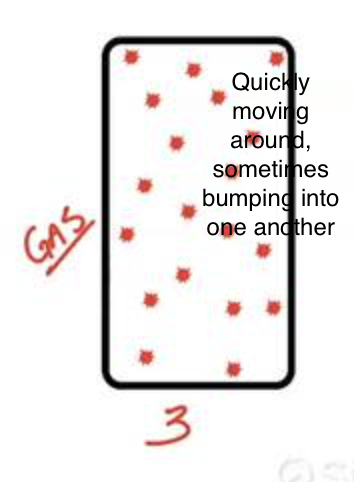
What is a gas? (volume, shape, flow, compressible, IM forces)
Matter that takes the shape of its container and fills the entire volume of its container, particles are far apart, and it is very compressible. Particles move past each other in order to flow, with lots of space in between them. No intramolecular forces.
Law of conservation of mass
Mass is neither created nor destroyed during ordinary chemical reactions or physical changes
Law of conservation of energy
Energy cannot be created or destroyed
What is an atom?
Smallest unit of an element that still has the same chemical identity as the element
What is an element?
Pure substance that cannot be broken down into simpler stable substances; made up of 1 type of atom.
What is a compound?
Pure substance made of two or more atoms of different elements joined by chemical bonds
What is a heterogenous mixture?
Mixture that does not blend smoothly throughout, and in which the individual substances stay distinct
What is a homogenous mixture?
Mixture that has constant composition throughout; always has a constant phase
What is a pure substance?
Substance with a uniform and unchanging composition, with only one type of particle
What is an intensive property, and examples?
A characteristic that does not depend on the amount of substance (e.g, density, color, and boiling point, melting point, conductivity, etc)
What is an extensive property and examples?
A characteristic that depends on the amount of matter (e.g mass, volume, amount of energy, concentration, etc)
What is a physical property and examples?
Characteristic that can be observed or measured without changing the sample’s composition, so the substance stays the same (e.g, color, density, odor, hardness, melting point, boiling point, etc)
What is a chemical property and examples?
Characteristic of a substance’s ability to go through changes that will produce a different substance (e.g, rusting, oxidation, flammability, reactivity, toxicity, etc.)
What is a physical change and an example?
Alters substance without changing chemical composition (ex: shattering glass)
What is a chemical change?
Process involving one or more substances being changed into a new one (AKA chemical reaction), always changes properties
What is a phase change and examples?
A type of physical change where matter transitions from one state to another (e.g melting, freezing, etc.)
Solid to liquid
Melting
Liquid to solid
Freezing
Solid to gas
Sublimation
Gas to solid
Deposition
Gas to liquid
Condensation
Liquid to gas
Vaporization
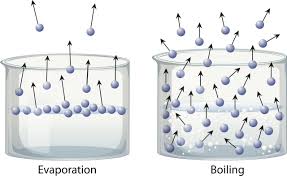
Types of vaporization and how they work
Evaporation - Sun adds kinetic energy to molecules at the top of water, causing them to move faster, break attractive forces, and become a gas
Boiling - Heat is added directly. Gas forms within the liquid, then escapes as bubbles rise to the surface.
What is a phase?
Any part of a system that has uniform properties and composition
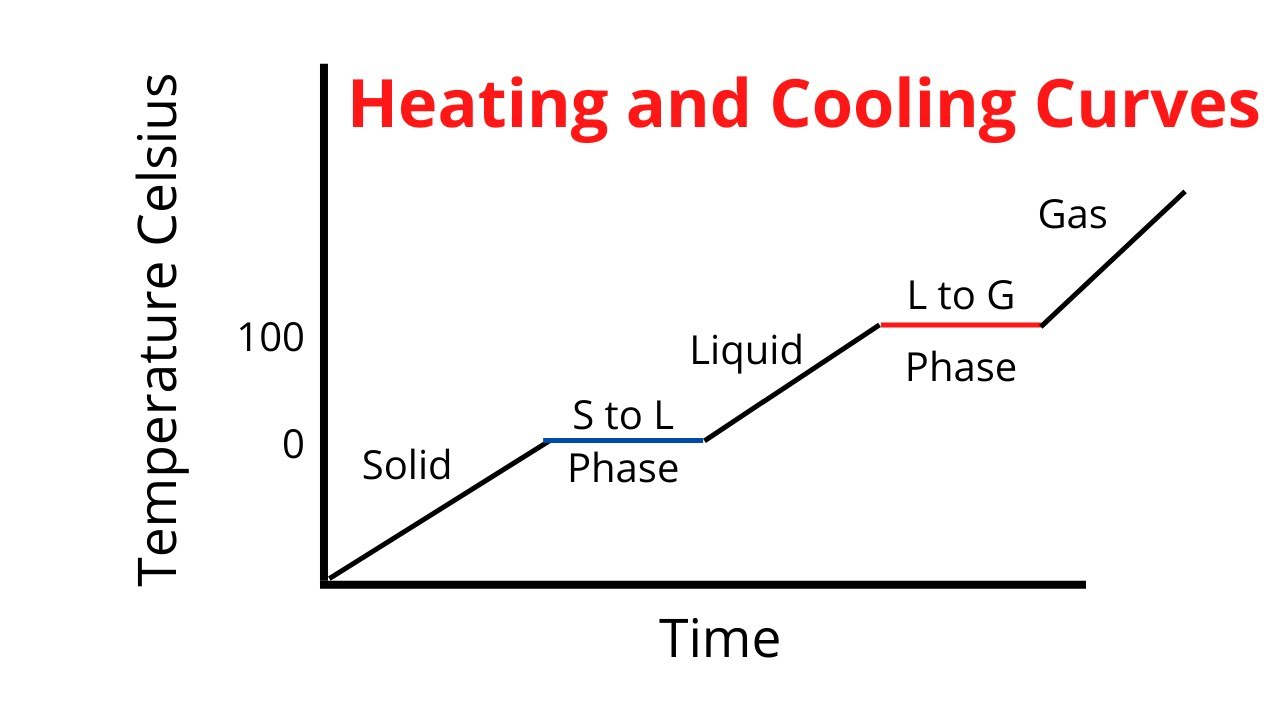
What is a heat curve diagram?
Graph showing how a substance changes as it is heated
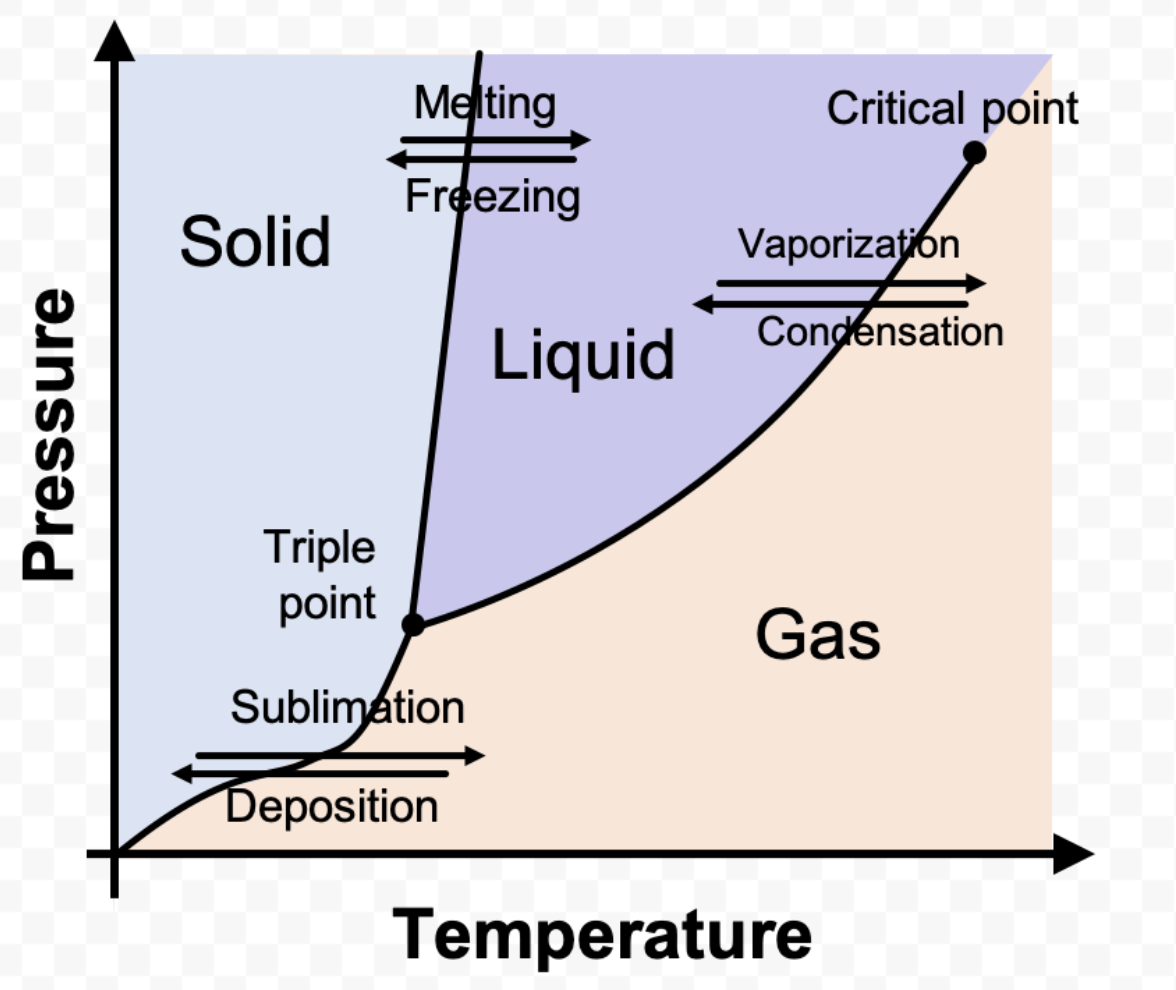
What is a phase diagram?
A graph of pressure vs. temperature that shows the conditions under which the phases of a substance exist
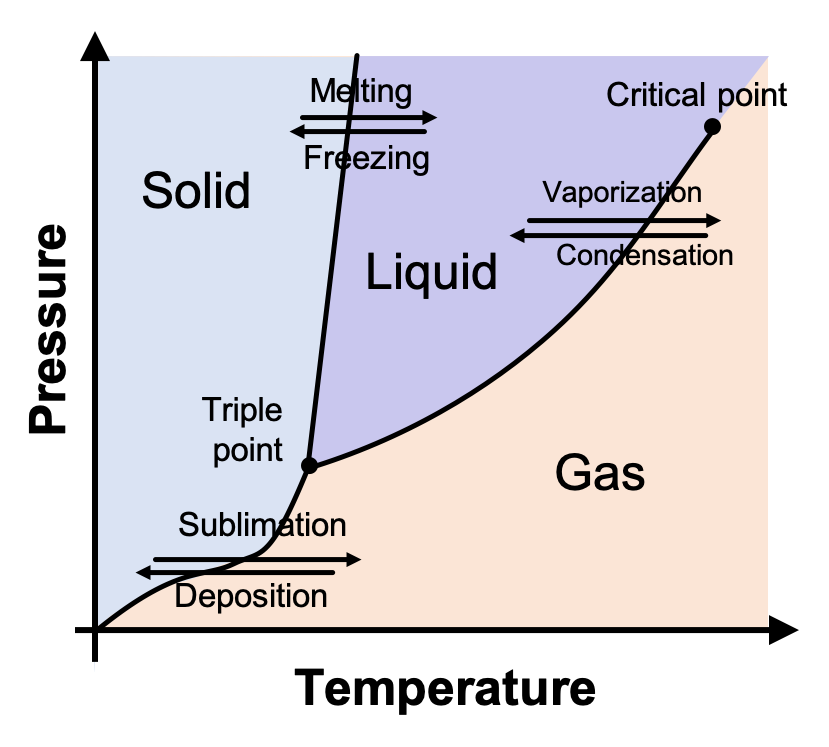
What is a triple point?
Temperature and pressure conditions where solid, liquid, and vapor can exist at equilibrium
What is a critical point?
Shows the temperature and pressure where a substance can no longer exist as a liquid
4 signs of a chemical change
Transfer of energy
Color change*
Gas production (besides boiling)
Precipitate formation (liquid + liquid = solid, think milk + vinegar)
Law of conservation of mass/energy in a candle
Mass: Wax (hydrocarbons) + O₂ → CO₂ + H₂O
The candle looks smaller, but atoms aren’t lost — they turn into gases that float away.
Energy: Chemical energy in wax → light + heat energy
Energy isn’t destroyed, just transformed.
Law of conservation of mass/energy in chromatography
Mass: The ink/pigment mixture doesn’t disappear — molecules just separate as they move with the solvent. Each pigment’s atoms are still present, just spread out on the paper.
Energy: No energy is destroyed — it’s mainly the solvent’s kinetic energy (movement of molecules) that carries pigments along.
Kinetic Molecular Theory
All matter is made out of particles that are in constant motion
Assumptions about gases with KMT
Gas particles are round and don’t take up space
Gas particles aren’t attracted to each other (no IM forces)
Gas particles move in constant, random motion
Gas particle collisions are perfectly elastic (no KE lost)
What are allotropes? (type of solid)
-Allotropes are two or more different molecular forms of the same element in the same physical state
-This can result in very different physical properties, such as diamond and graphite for carbon
What are crystals, and examples? (type of solid)
Crystals are materials whose particles are arranged in an orderly, repeated 3D pattern. (e.g, sugar, snowflakes)
Relationship between kinetic molecular theory and temperature (3)
-As temperature increases, the average kinetic energy of particles also increases.
-Temperature is determined by a substance’s average KE of all the molecules.
-KE is directly proportional to Kelvin.
What are amorphous solids, and examples?
Amorphous solids are solids without a crystal form; they lack an internal structure (e.g glass, quartz)
What are unit cells?
Unit cells are the smallest repeating units in a crystal that define the crystal structure.
What is diffusion? (property of gas)
Diffusion is the tendency of gas particles to move from a higher concentration to a lower concentration, until those concentrations are equal.
What is effusion? (property of gas)
Effusion is the process by which gas particles escape through a tiny opening, typically occurring at a lower pressure.
What is gas pressure & what is it caused by? (property of gas)
The sum of the forces exerted on the surface area of an object, caused by different gas/air molecules colliding with a surface.
What is a vacuum?
An empty space with no particles and therefore no pressure.
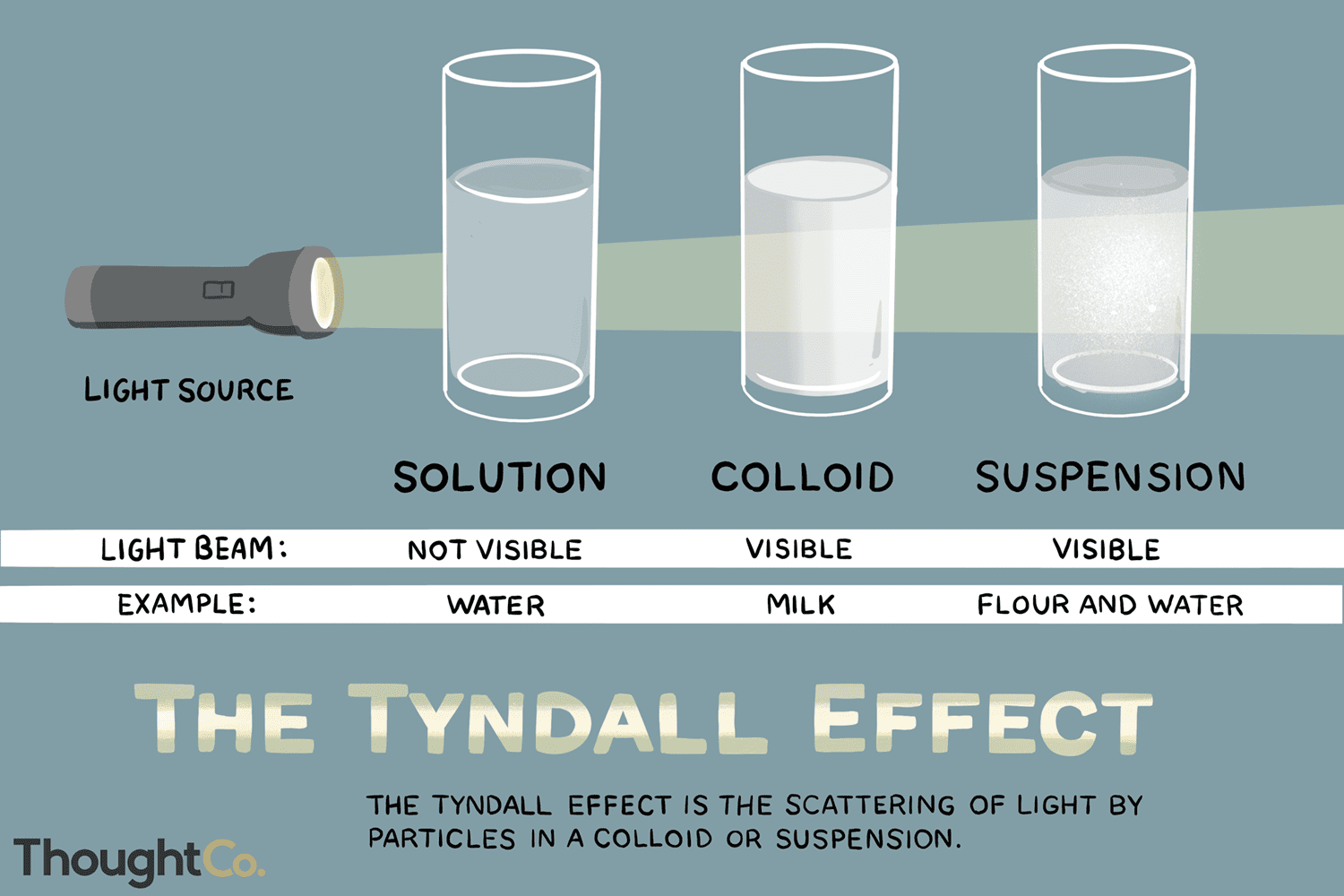
What is the Tyndall Effect?
The scattering of visible light by particles in a colloid or suspension. (ex: light coming through trees). It leads to the visibility of light beams in certain conditions.
What is Brownian motion? (property of aqueous solution)
The chaotic and random movement of colloidal particles, caused by the collision of microscopic particles with fast-moving particles.
What is coagulation?
The clumping of charged colloidal particles with other charged particles.
What is emulsion?
A type of colloid where polar and non-polar molecules are brought together with the help of an additional particle, an emulsifying agent (e.g mayo = oil + vinegar + egg, milk = oil + water + proteins)
What is a colloid, and examples?
A heterogenous mixture made of large and small particles spread throughout that don’t settle out. Can include any phase (e.g fog, milk)
What is a suspension?
A mixture where particles of one material are much larger (also larger than particles in a colloid) than particles of the other material and can settle out over time. Often includes solid and liquid particles (e.g flour and water).
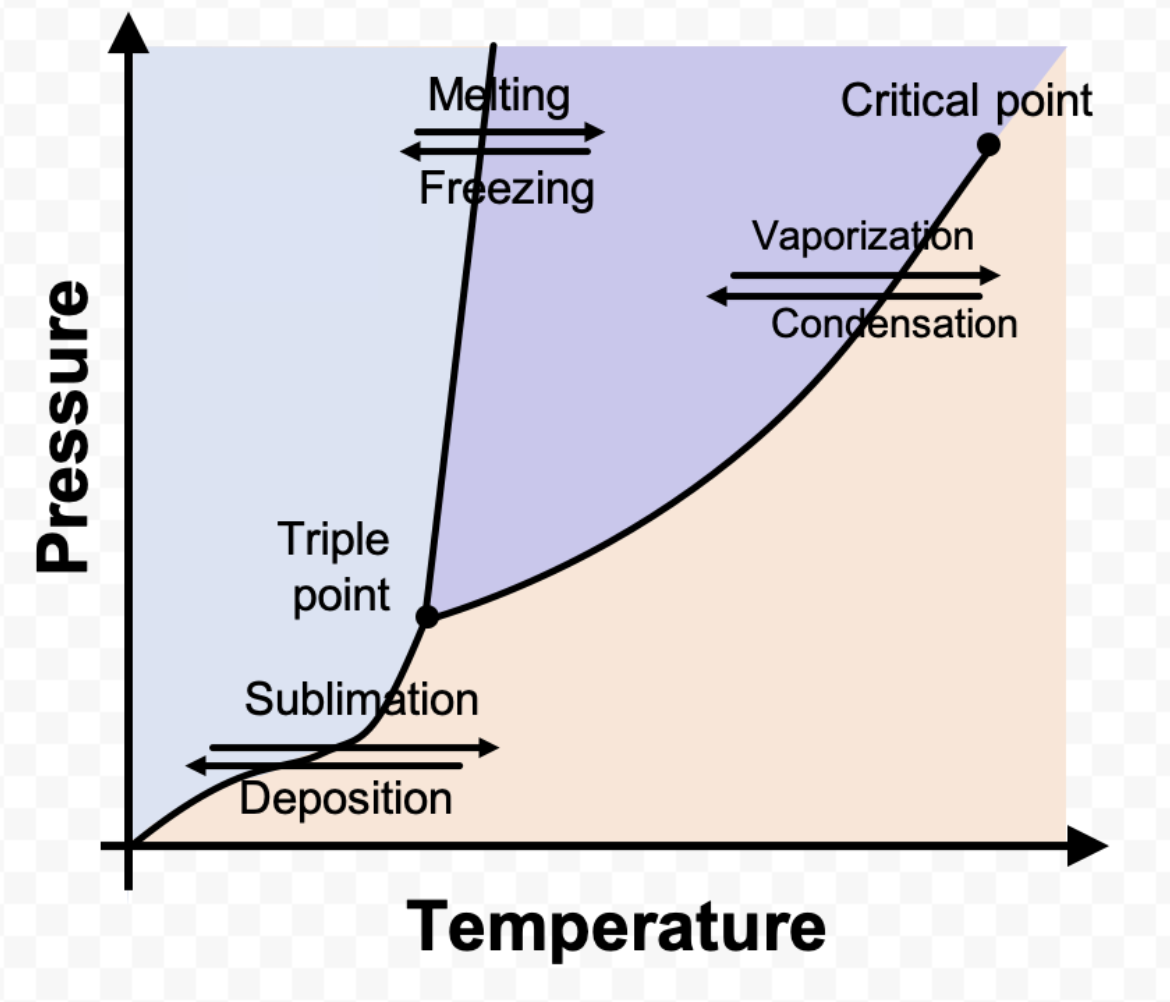
What is each area in a phase diagram?
What is each area in a heat curve?
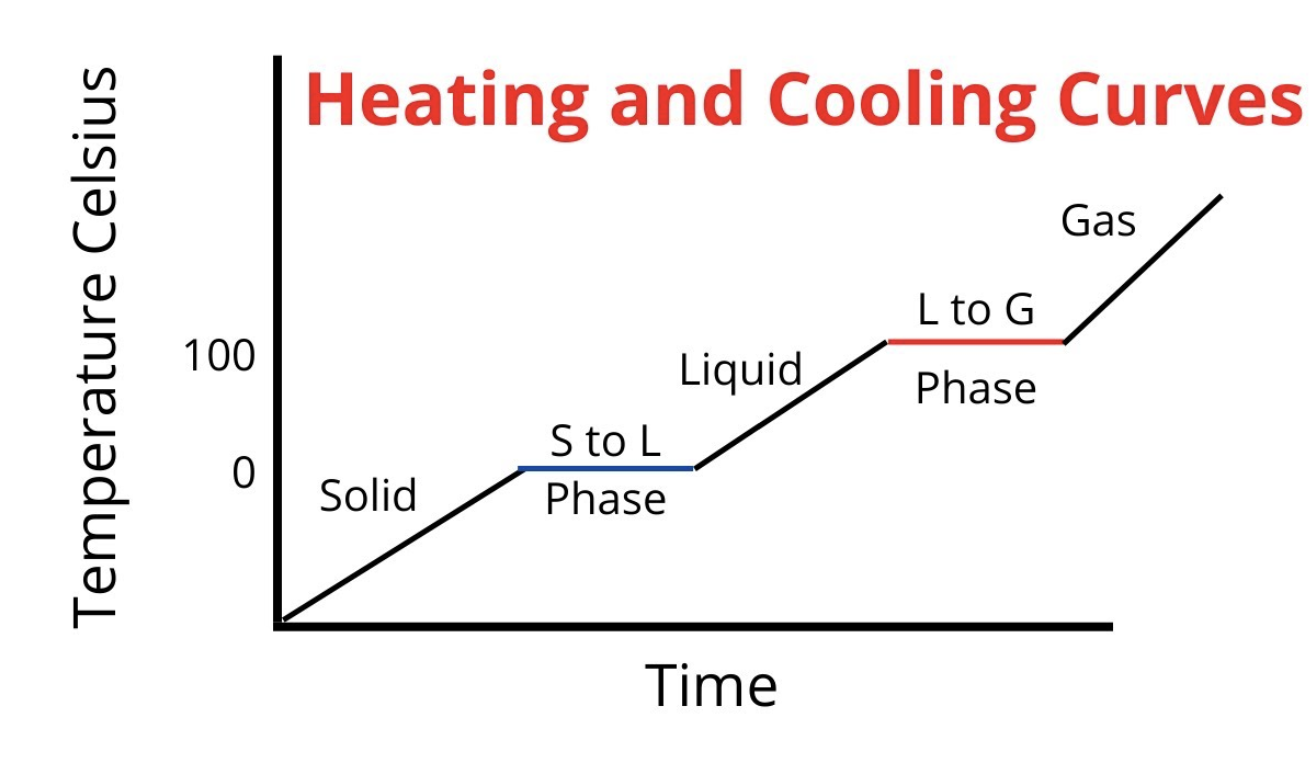
What is a solution?
Where one substance (solute) is completely dissolved in another (solvent). It can also be known as a homogenous mixture.
As volume decreases, pressure
increases and vice versa (inverse relationship)

As you heat a gas, the volume
increases (direct relationship)
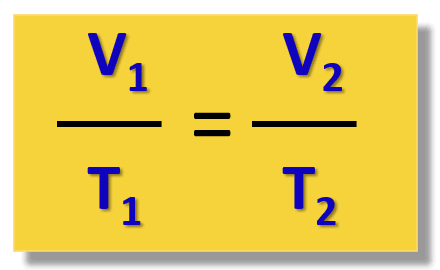
At a constant volume, as temperature increases, pressure
increases (direct relationship)
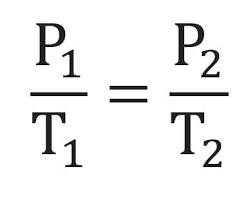
Combined Gas Law
Combines the relationships of the previous three gas laws
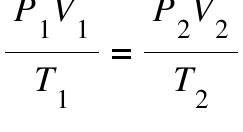
Standard Temperature and Pressure (STP)
is 0 degrees Celsius (273 K) and 1 atmosphere of pressure.
Ideal gas constant
0.0821 L·atm/(mol·K), used for R
Ideal gas law (what is it + equation)
Describes behavior of an ideal gas. Equation: PV=nRT.
R = ideal gas constant
What is specific heat?
Specific heat is the amount of heat required to raise the temperature of one gram of a substance by one degree Celsius. It varies with the material. Equation: Q = McΔT.
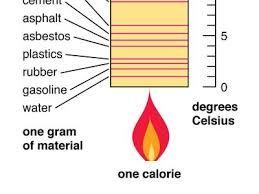
Having a lower specific heat capacity means that
a material requires less energy to increase its temperature, causing it to heat up and cool down quickly.
Temperature must be converted to Kelvin (+ 273) for
all gas laws.
For the ideal gas law,
temperature must be Kelvin, volume must be L, pressure should be atm, and n should be in moles.