13. Neuromuscular Transmission
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
22 Terms
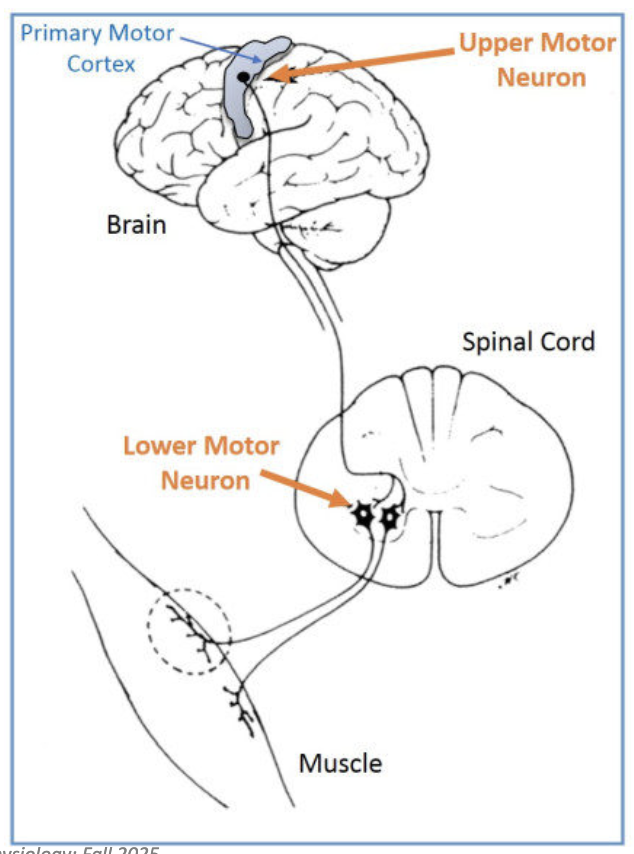
What controls voluntary muscle contractions?
Motor neurons control contraction
Primary motor cortex coordinates all voluntary contractions
Signals travel via upper and lower motor neurons
Upper motor neurons synapse with other motor neurons
Lower motor neurons synapse with the muscle
Final outcome → muscle contraction
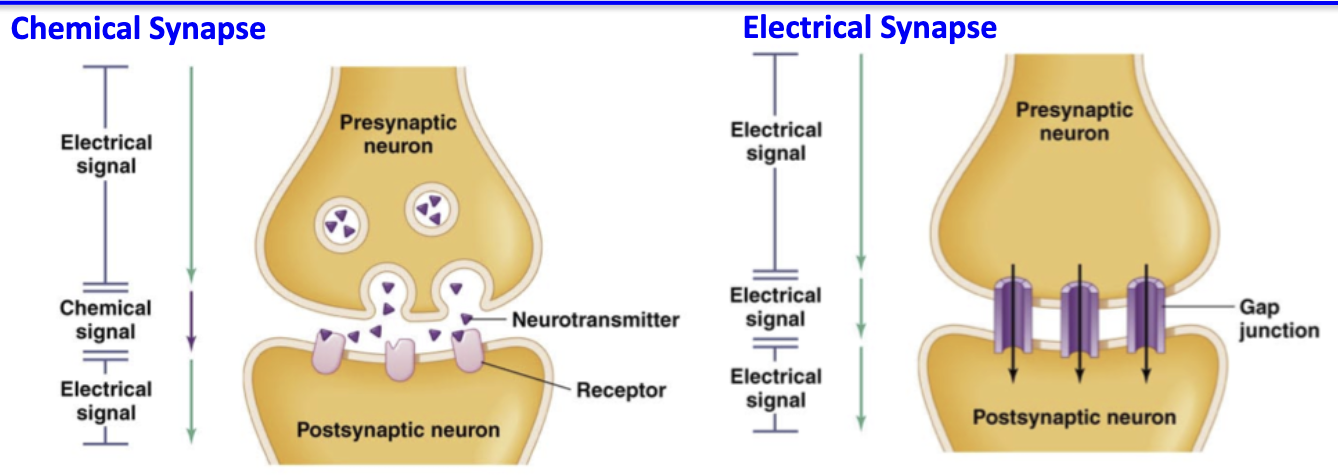
What are the two types of synapses and how do they differ?
1. Chemical synapses
Found between neurons or at the NMJ
Neurotransmitters released from presynaptic neuron → bind postsynaptic receptors
Signal transduction: electrochemical → chemical → electrochemical
Slower due to chemical step
2. Electrical synapses
Rare, mostly in the brain
Direct flow of electrical current through gap junctions
Faster, no signal conversion required
How does neuronal signalling achieve convergence?
Convergence = multiple presynaptic inputs integrated by a postsynaptic neuron
Occurs via neuronal pools
Brain has >1 billion neurons and >1 trillion synapses, allowing massive integration
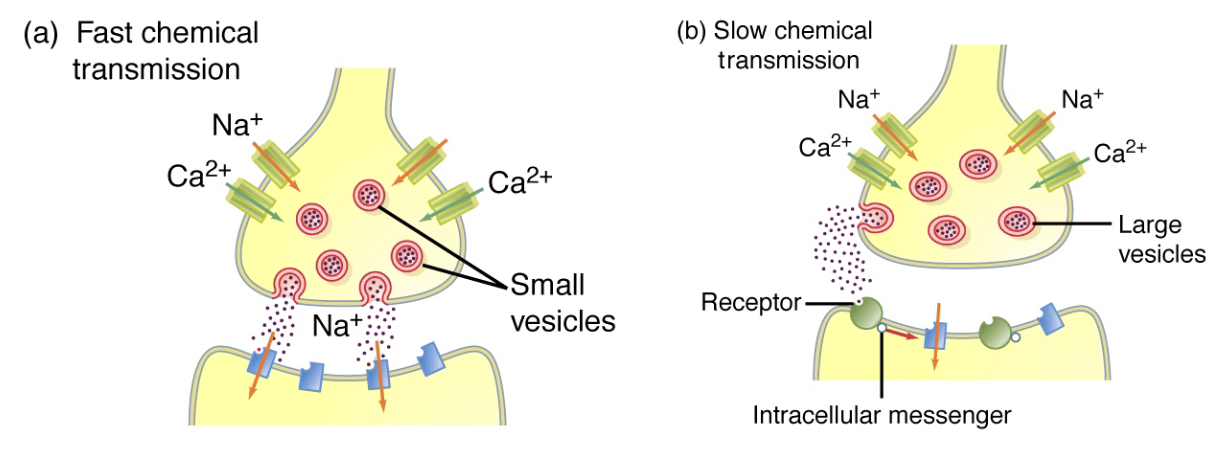
What are the two modes of chemical transmission and how do they differ?
1. Fast transmission (ionotropic)
Neurotransmitter binds ligand-gated ion channels → channel opens directly
Rapid response
Example: increase in Ca²⁺ triggers vesicle release at synapse
Receptor = channel
2. Slow transmission (metabotropic)
Neurotransmitter binds G-protein-coupled receptor → activates intracellular signaling → ion channels and other processes affected
Relies on secondary messengers
Slower, indirect effect
Note:
Single neurons can use both modes
Single neurotransmitter can act both ways
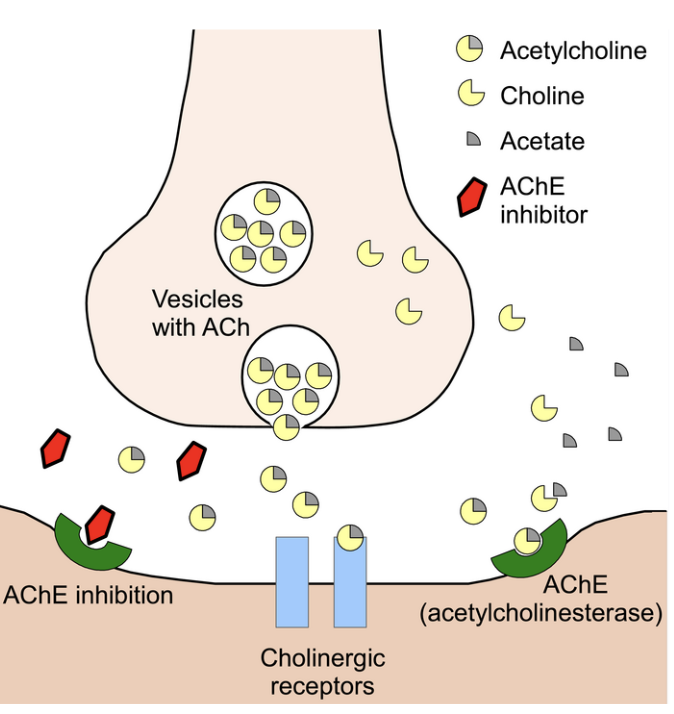
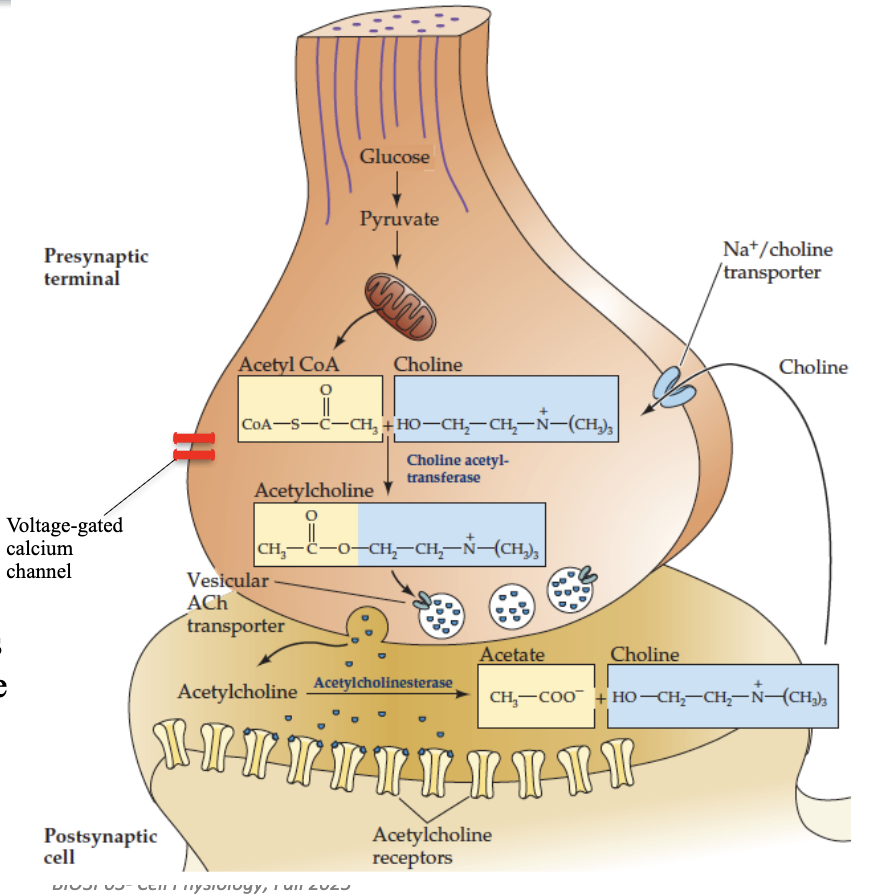
How do chemical synapses function at the neuromuscular junction (NMJ)?
Presynaptic terminal contains vesicles storing neurotransmitter (ACh)
Action potential arrives → vesicles release ACh into synaptic cleft
Postsynaptic receptors bind ACh → ion channels open → change in membrane permeability → depolarization
Acetylcholinesterase breaks down ACh → prevents sustained signal
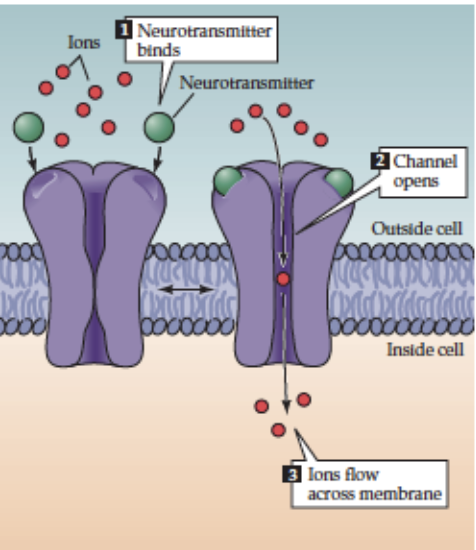
How does acetylcholine act via fast transmission?
Receptor: Nicotinic ACh receptor (nAChR)
Transmembrane protein complex, 5 subunits (2α subunits bind 1 ACh each)
Location: NMJ & postganglionic cells of vertebrate autonomic NS
Mechanism: ACh binding → direct opening of ligand-gated ion channels → rapid depolarization
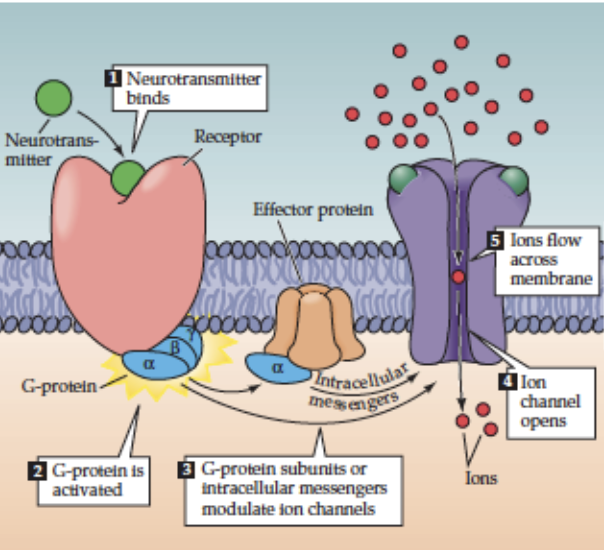
How does acetylcholine act via slow transmission?
Receptor: Muscarinic ACh receptor (mAChR) → G-protein-coupled receptor (GPCR)
Mechanism: ACh binds → GPCR activates G-protein on cytoplasmic side → indirectly opens ion channels (via second messengers like cAMP)
Location: target cells of parasympathetic NS (e.g., cardiac tissue) in vertebrates
Effect: slower, indirect modulation of postsynaptic cell activity
What happens at the NMJ when an action potential arrives?
Presynaptic AP → release millions of ACh molecules into synaptic cleft
Muscle depolarizes → opens voltage-gated Na⁺ & K⁺ channels → postsynaptic action potential
1 neuron → 1 muscle fiber → organized contraction
Motor end plate = postsynaptic region of NMJ with high density of nAChRs; only here
Outside motor end plate: mostly voltage-gated Na⁺ & K⁺ channels, like in neurons
Graded end plate potential (EPP) → triggers AP; more ACh → more channels open → larger EPP → stronger depolarization
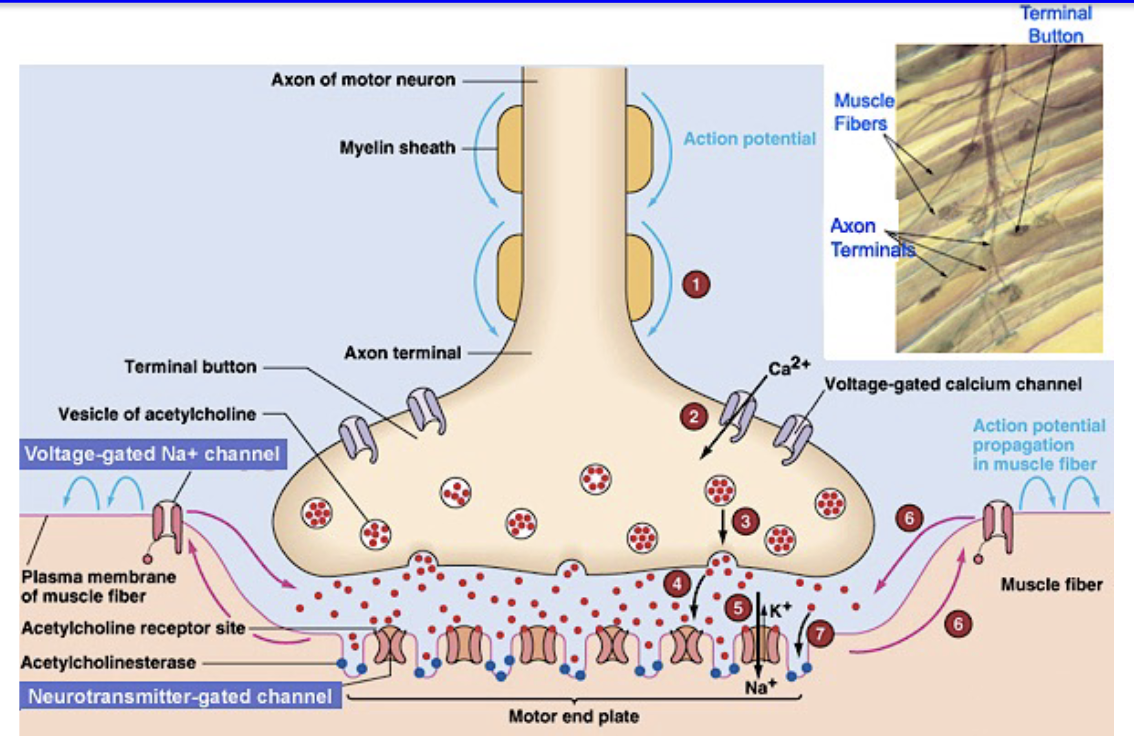
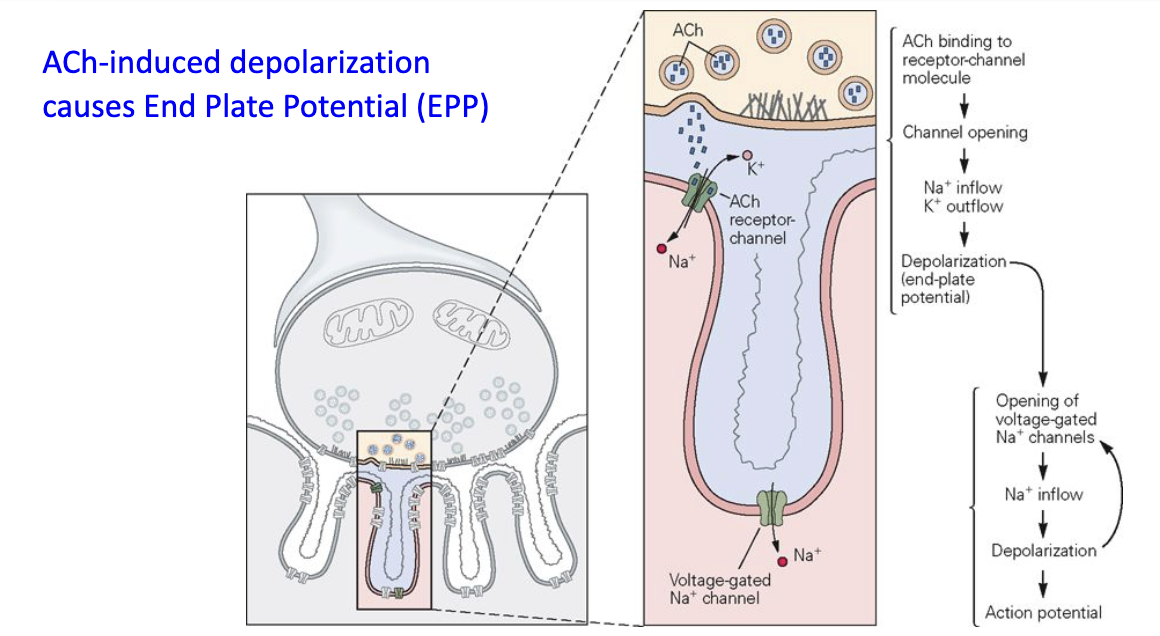
What is the End Plate Potential (EPP)?
ACh binds to AChR → opens ligand-gated channels → muscle depolarization
Depolarization opens nearby voltage-gated Na⁺ channels
If threshold reached → muscle action potential
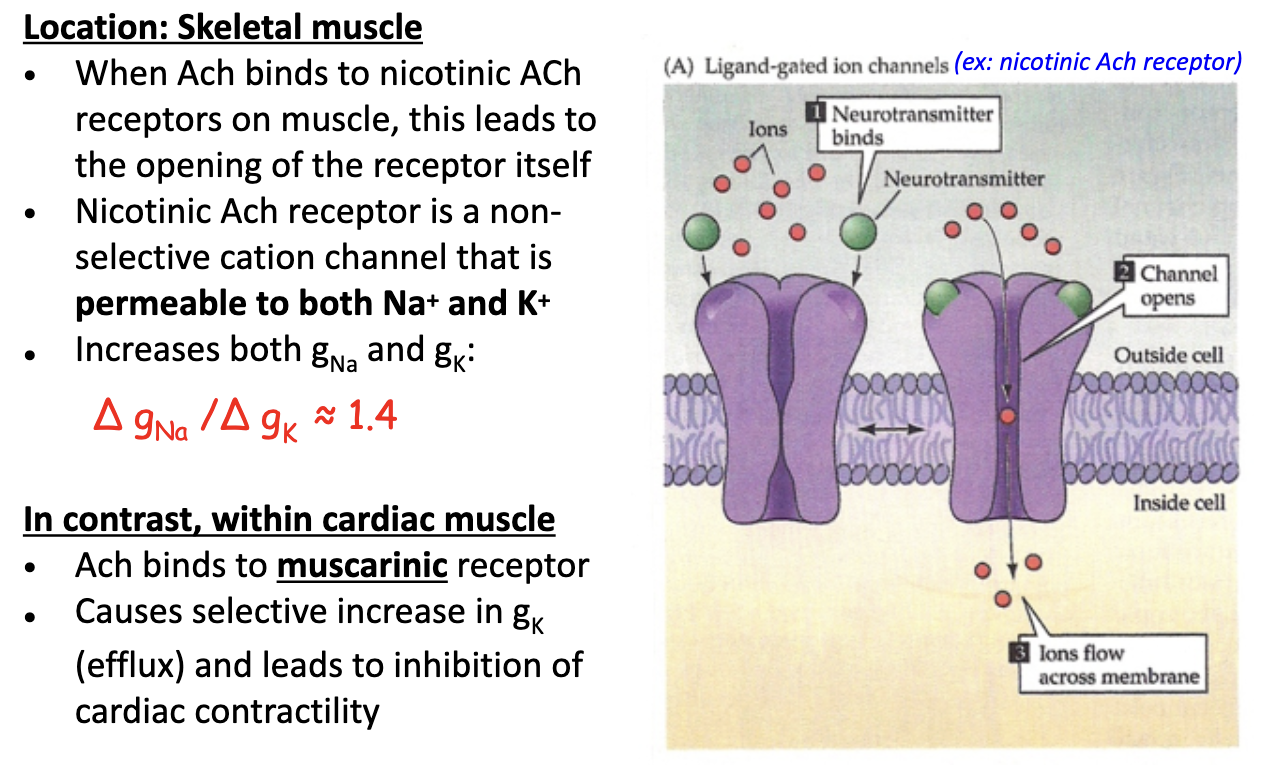
How do nicotinic ACh receptors function in skeletal vs. cardiac muscle?
Skeletal muscle
nAChR = non-selective cation channel (permeable to Na⁺ & K⁺)
ΔgNa / ΔgK ≈ 1.4 → more Na⁺ influx → depolarization
Mediated by sympathetic NS
Cardiac muscle
ACh binds muscarinic (metabotropic) receptor
↑ gK efflux → hyperpolarization → inhibits contractility
Mediated by parasympathetic NS
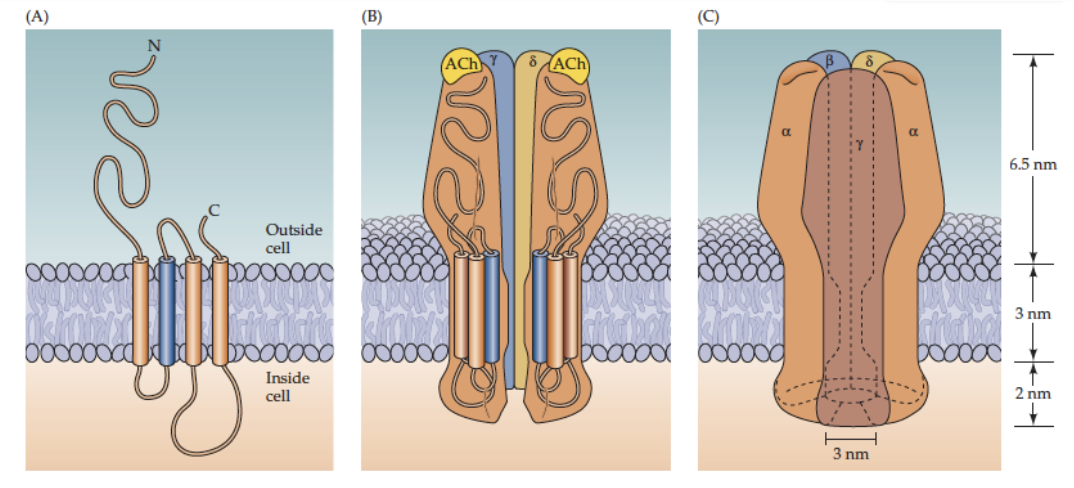
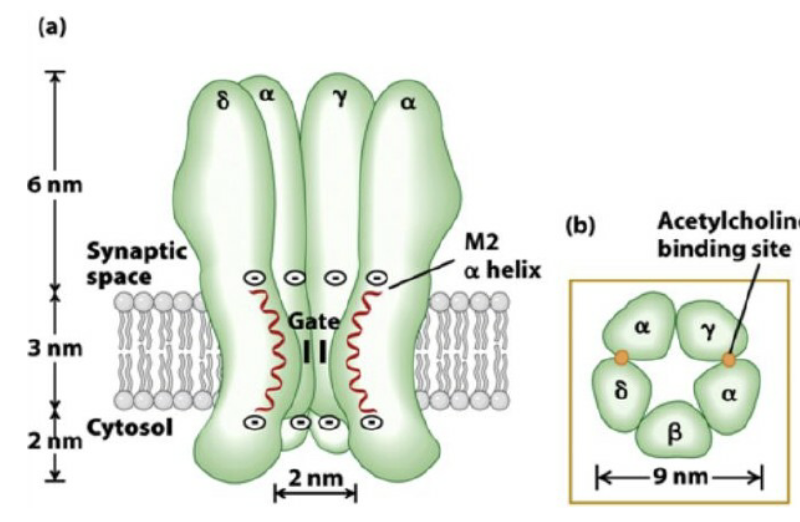
What is the structure of the nicotinic ACh receptor?
ONLY TESTABLE PART: 2α subunits actively bind ACh, permeable to sodium and potassium (higher for sodium)
NOT-TESTABLE:
5 subunits: 2α, 1β, 1γ, 1δ at skeletal NMJ
Each subunit crosses membrane 4 times (M1–M4)
Openings at either end are large ~2-3nm in diameter, narrowest region ~-0.6nm (compare this to Na+ and K+ ion which are <0.3nm)
Central pore formed by 5 subunits, M2 (blue region) important for ion translocation
How does the nAChR select for cations?
M2 region rings contain negatively charged amino acids at the mouth of the channel (inside & outside)
→ These attract and concentrate positively charged cations (Na⁺, K⁺)
Pore lining: uncharged polar amino acids (e.g., -OH, -NH₂ groups)
→ Provide a selective path for cations to pass throughOverall mechanism: combination of electrostatic attraction at channel entrances and polar lining inside pore ensures cation selectivity
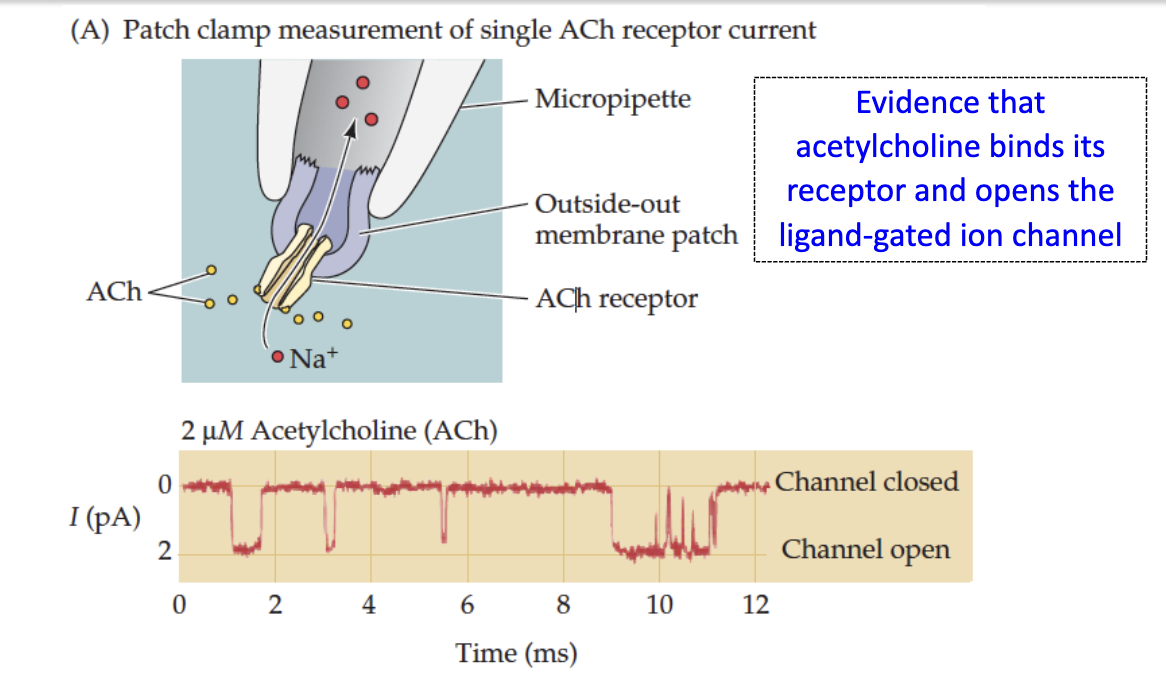
How can we measure nAChR activation?
Outside-out patch clamp: electrode in ACh solution, measures current through single receptor → shows ligand-gated channel opens
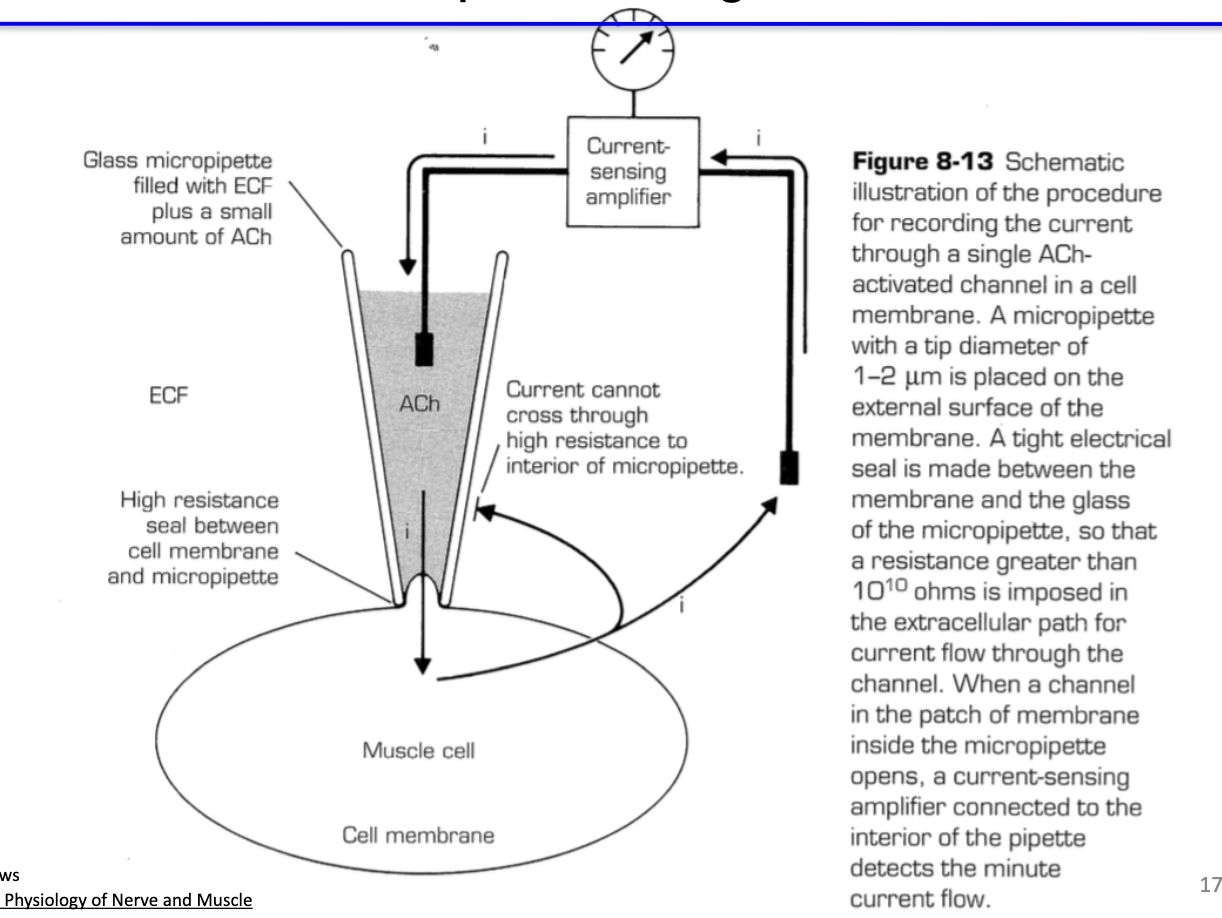
What is the on-cell patch configuration and why is it used to measure nAChR activity?
On-cell patch clamp: measures receptor activity in intact cell membrane
Limitation: cannot dynamically change ACh concentration in pipette → typically use standard ACh concentration
Advantage: more physiologically relevant, preserves cytosolic environment and secondary signaling molecules, important for studying metabotropic receptors like muscarinic AChRs
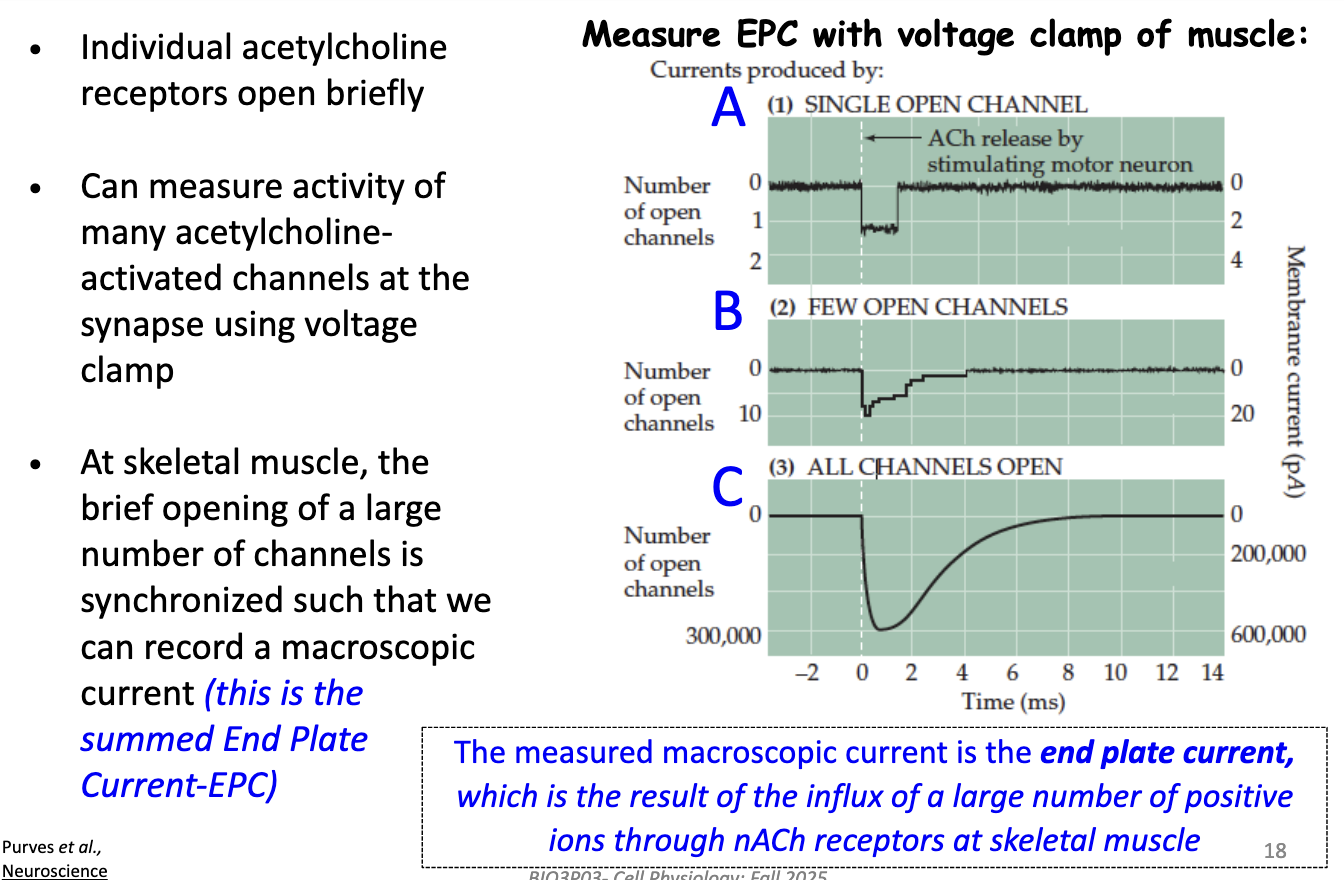
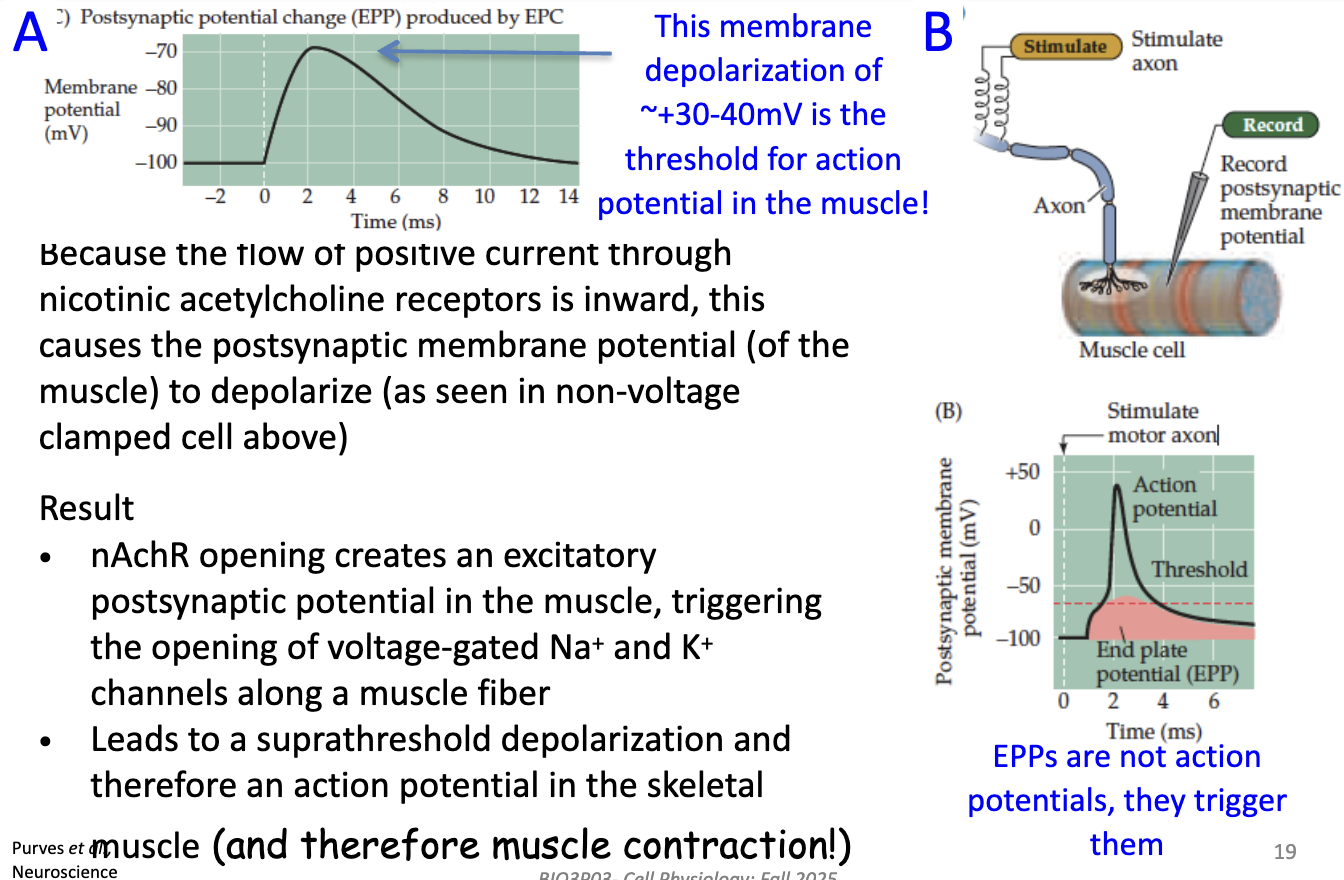
How are macroscopic currents recorded in a muscle fiber, and what does the End Plate Current (EPC) represent?
Individual nACh receptors open briefly when activated by ACh
Using a voltage clamp, the activity of many receptors at the synapse can be measured
In skeletal muscle, the brief openings are synchronized → produce a macroscopic current
This summed current is called the End Plate Current (EPC)
EPC reflects the influx of many positive ions (Na⁺) through nACh receptors, leading to postsynaptic depolarization
What is the End Plate Potential (EPP) and how does it lead to muscle contraction?
EPP = depolarization of muscle membrane ~+30–40 mV
→ This is threshold for muscle action potential
Mechanism: inward positive current through nACh receptors depolarizes postsynaptic membrane
EPP ≠ action potential; it triggers AP by opening voltage-gated Na⁺ & K⁺ channels along the muscle fiber
Result: suprathreshold depolarization → muscle action potential → contraction
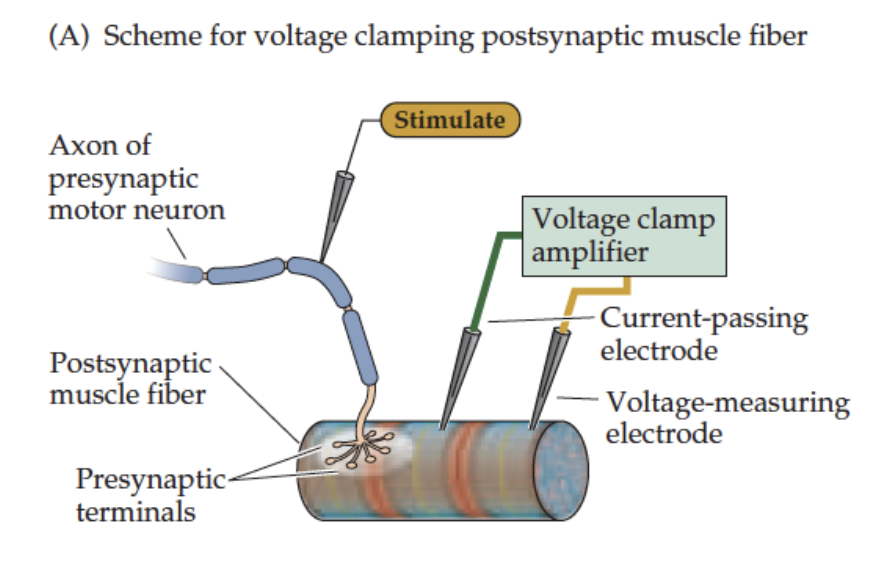
How can we identify which ions flow during the EPC?
Voltage clamp technique is used:
Voltage clamp the muscle fiber using 2 electrodes
Simultaneously stimulate the presynaptic motor neuron → ACh release
Record macroscopic EPCs in response to ACh
This allows determination of ion fluxes (Na⁺, K⁺) through nACh receptors at the NMJ
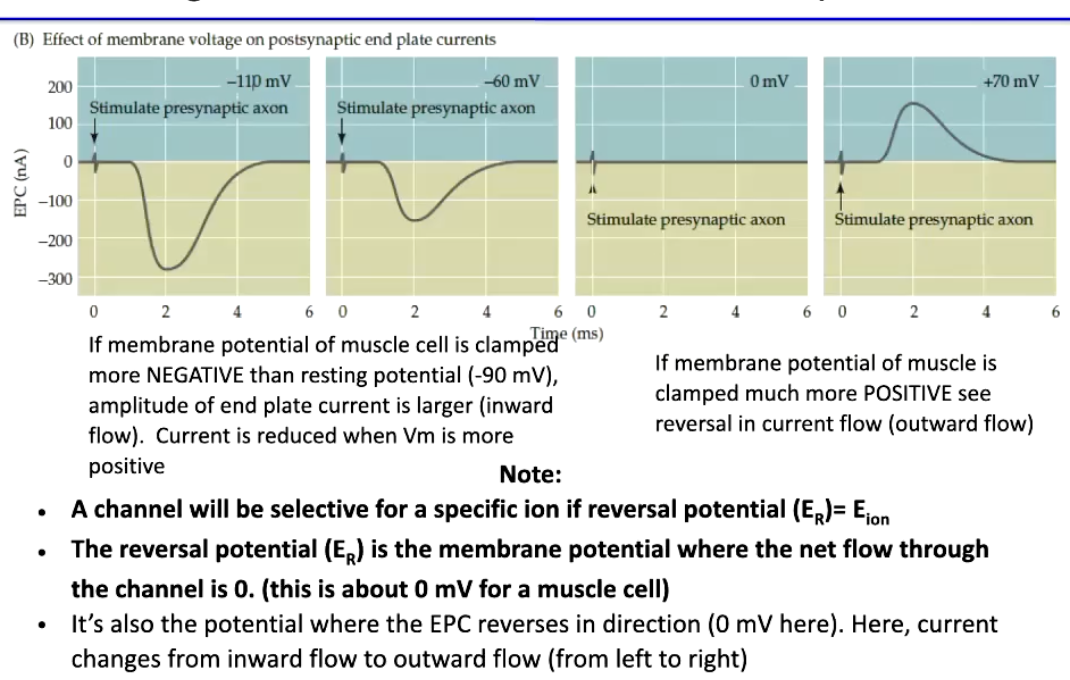
How does membrane potential affect the End Plate Current (EPC) in skeletal muscle, and what is the reversal potential?
EPC amplitude & polarity depend on membrane potential (Em):
Em more negative than resting (-90 mV) → larger inward EPC (stronger Na⁺ influx)
Em more positive than resting → smaller EPC; at very positive potentials → outward current (K⁺ efflux dominates)
Reversal potential (ER) = Em where net current = 0
For nAChR, ER ≈ 0 mV
At ER, EPC reverses direction (inward → outward)
Key point: ER indicates channel ion selectivity; current direction depends on Na⁺ and K⁺ driving forces
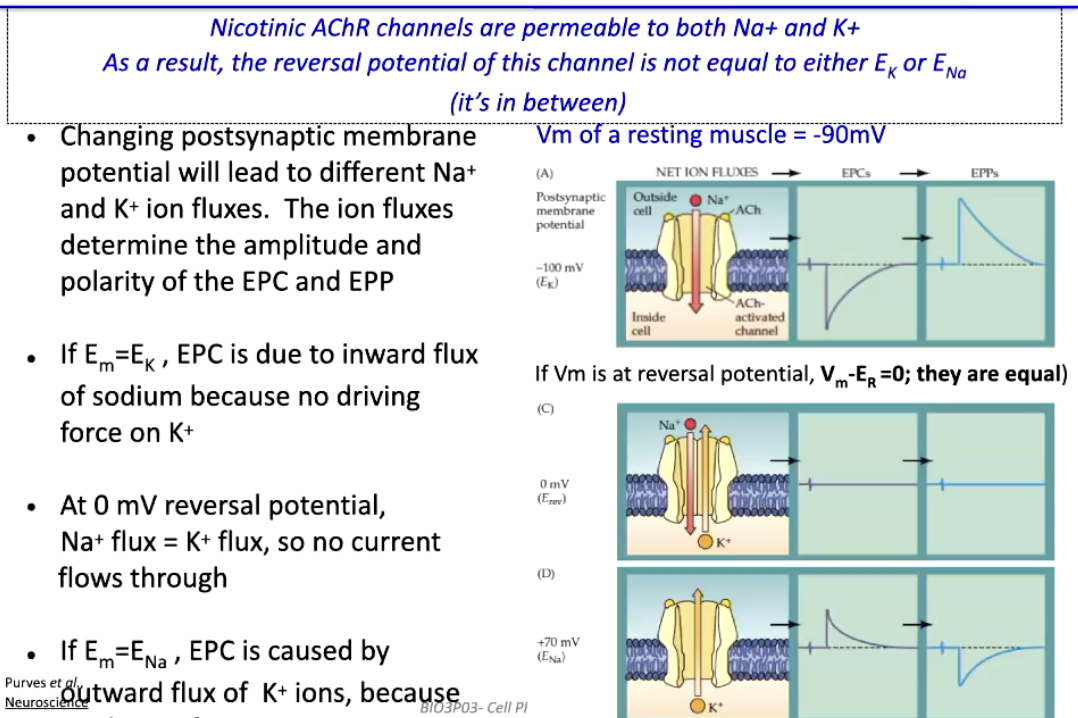
How can we determine which ions flow during the End Plate Current (EPC) through nicotinic ACh receptors?
nAChR channels are permeable to both Na⁺ and K⁺
Reversal potential (ER) is between ENa and EK, not equal to either
Changing membrane potential (Em) alters Na⁺ & K⁺ flux → affects amplitude & polarity of EPC/EPP
Em = EK → K⁺ has no driving force → EPC is inward Na⁺ current
Em = 0 mV (ER) → Na⁺ flux = K⁺ flux → no net current
Em = ENa → Na⁺ has no driving force → EPC is outward K⁺ current
Resting muscle potential: Vm ≈ -90 mV → inward Na⁺ dominates EPC
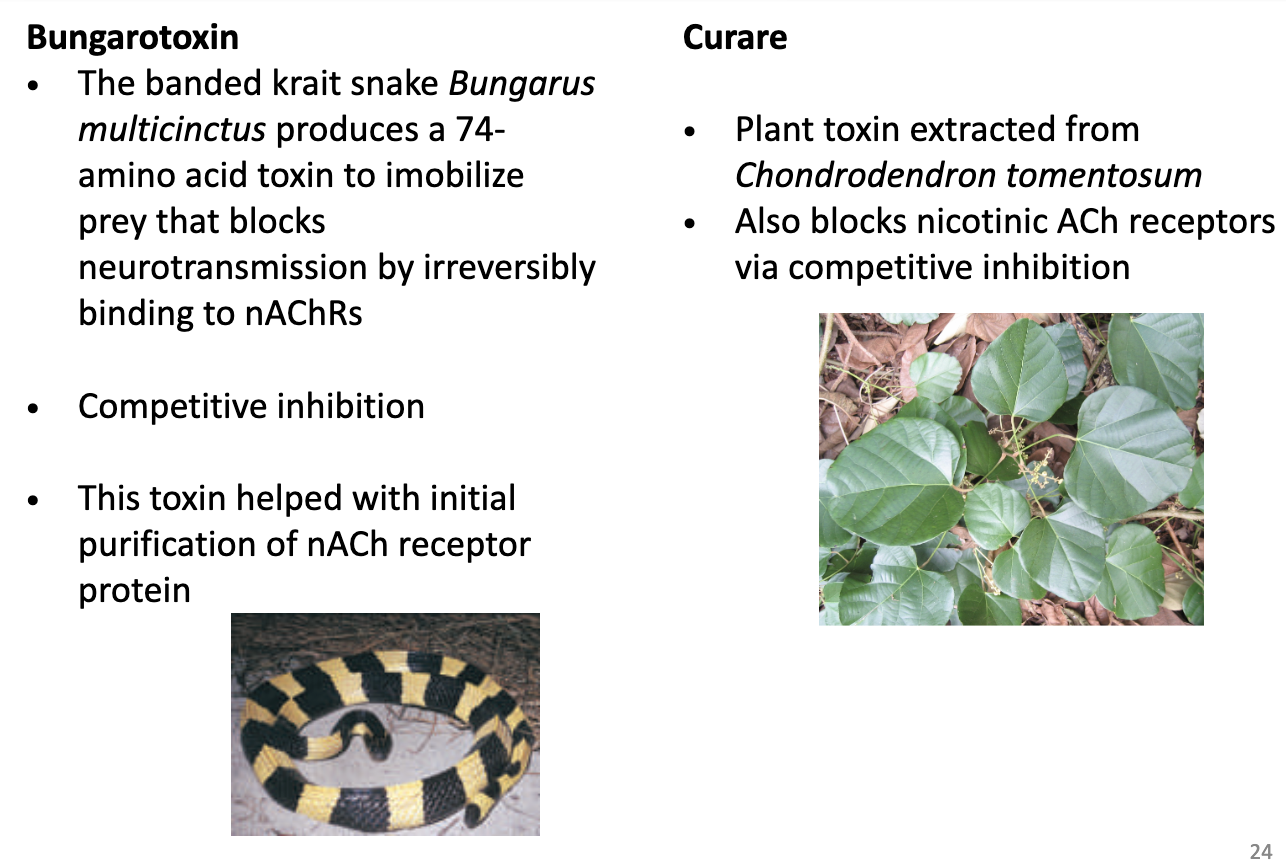
What are common probes for studying muscle nicotinic ACh receptors and how do they work?
Bungarotoxin
From banded krait snake (Bungarus multicinctus), 74-amino acid toxin
Irreversibly binds nAChRs → blocks neurotransmission
Competitive inhibitor
Used for initial purification of nACh receptor protein
Curare
Plant toxin from Chondrodendron tomentosum
Blocks nAChRs via competitive inhibition
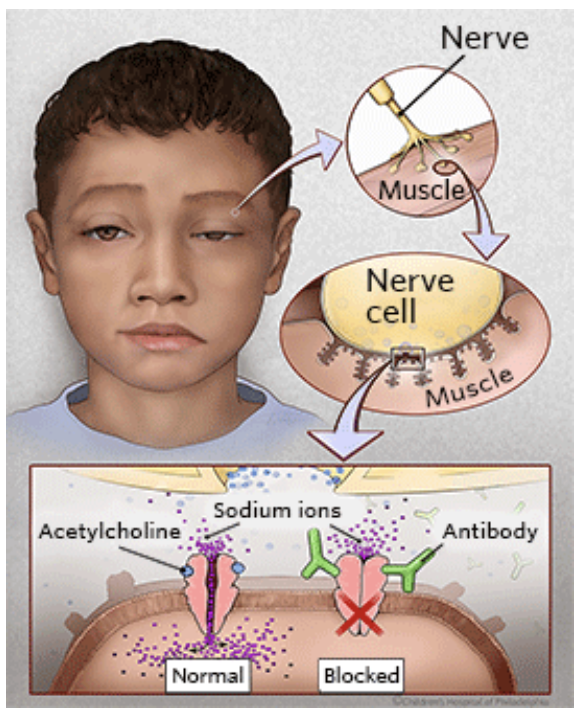
What is myasthenia gravis and how does it affect the neuromuscular junction?
Chronic neuromuscular disease → causes skeletal muscle weakness
Mechanism: autoantibodies attack nicotinic ACh receptors (nAChRs)
Result: impaired neuromuscular transmission → reduced muscle contraction
How is myasthenia gravis treated pharmacologically?
Strategy: prolong ACh action at NMJ by reducing its breakdown
Drug example: Neostigmine → inhibits acetylcholinesterase
Result: more ACh available at nAChRs → improves muscle contraction