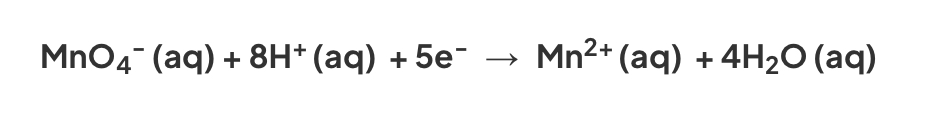Chemistry 2.5- Transition Metals
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
27 Terms
What is a transition metal? How is this different from a d-block metal?
Transition metals are elements that form at least one stable ion with an incomplete d-subshell
D-block elements have their valence electrons in the d-subshell
For example:
Iron has the electron arrangement [Ar] 4s2 3d6, and forms the ions Fe2+, [Ar] 3d6, and Fe3+, [Ar] 3d5, so it is both a d-block element and a transition metal
ZInc has the electron arrangement [Ar] 4s2 3d10, and forms the ion Zn2+, [Ar] 3d10, so it is a d-block element but not a transition metal
Scandium has the electron arrangement [Ar] 4s2 3d1, and forms the ion Sc3+, [Ar] 3d0, so it is a d-block element but not a transition metal
![<ul><li><p><strong>Transition</strong> metals are elements that form <strong>at least one stable ion with an incomplete d-subshell </strong></p></li><li><p><strong>D-block </strong>elements have their <strong>valence electrons in the d-subshell</strong></p></li></ul><p>For example:</p><ul><li><p>Iron has the electron arrangement [Ar] 4s<sup>2</sup> <strong>3d<sup>6</sup></strong>, and forms the ions Fe<sup>2+</sup>, [Ar] <strong>3d<sup>6</sup></strong>, and Fe<sup>3+</sup>, [Ar] <strong>3d<sup>5</sup></strong>, so it is <strong>both </strong>a d-block element and a transition metal</p></li><li><p>ZInc has the electron arrangement [Ar] 4s<sup>2 </sup><strong>3d<sup>10</sup></strong>, and forms the ion Zn<sup>2+</sup><span>, </span>[Ar] <strong>3d<sup>10</sup></strong>, so it is a d-block element but <strong>not</strong> a transition metal</p></li><li><p>Scandium has the electron arrangement [Ar] 4s<sup>2 </sup><strong>3d<sup>1</sup></strong>, and forms the ion Sc<sup>3+</sup><span>, </span>[Ar] <strong>3d<sup>0</sup></strong>, so it is a d-block element but <strong>not</strong> a transition metal</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/90ddbe69-a9be-46d1-88aa-a74f5426fd72.png)
What are the general properties of transition metals?
Can exist in variable oxidation states (eg. Na can only form Na+ whereas Fe can form Fe2+ and Fe3+)
Can form complex ions (forms co-ordinate bonds with several ions/molecules called ligands to form an ion, eg. [Cr(NH3)6]3+, [Cr(OH)6]3- and [Cr(H2O)6]3+)
Can form coloured compounds//ions
Can behave as catalysts
![<ul><li><p><strong>Can exist in variable oxidation states</strong> (eg. Na can only form Na<sup>+</sup> whereas Fe can form Fe<sup>2+</sup> <strong>and </strong>Fe<sup>3+</sup>)</p></li><li><p><strong>Can form complex ions</strong> (forms co-ordinate bonds with several ions/molecules called <strong>ligands</strong> to form an ion, eg. [Cr(NH<sub>3</sub>)<sub>6</sub>]<sup>3+</sup>, [Cr(OH)<sub>6</sub>]<sup>3-</sup> and [Cr(H<sub>2</sub>O)<sub>6</sub>]<sup>3+</sup>)</p></li><li><p><strong>Can form coloured compounds//ions</strong></p></li><li><p><strong>Can behave as catalysts</strong></p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/0e71923c-24a3-464f-9182-c460b319d3b3.png)
What is a Lewis acid? What is a Lewis base?
A Lewis acid is a species that can accept a lone pair
A Lewis base is a species that can donate a lone pair
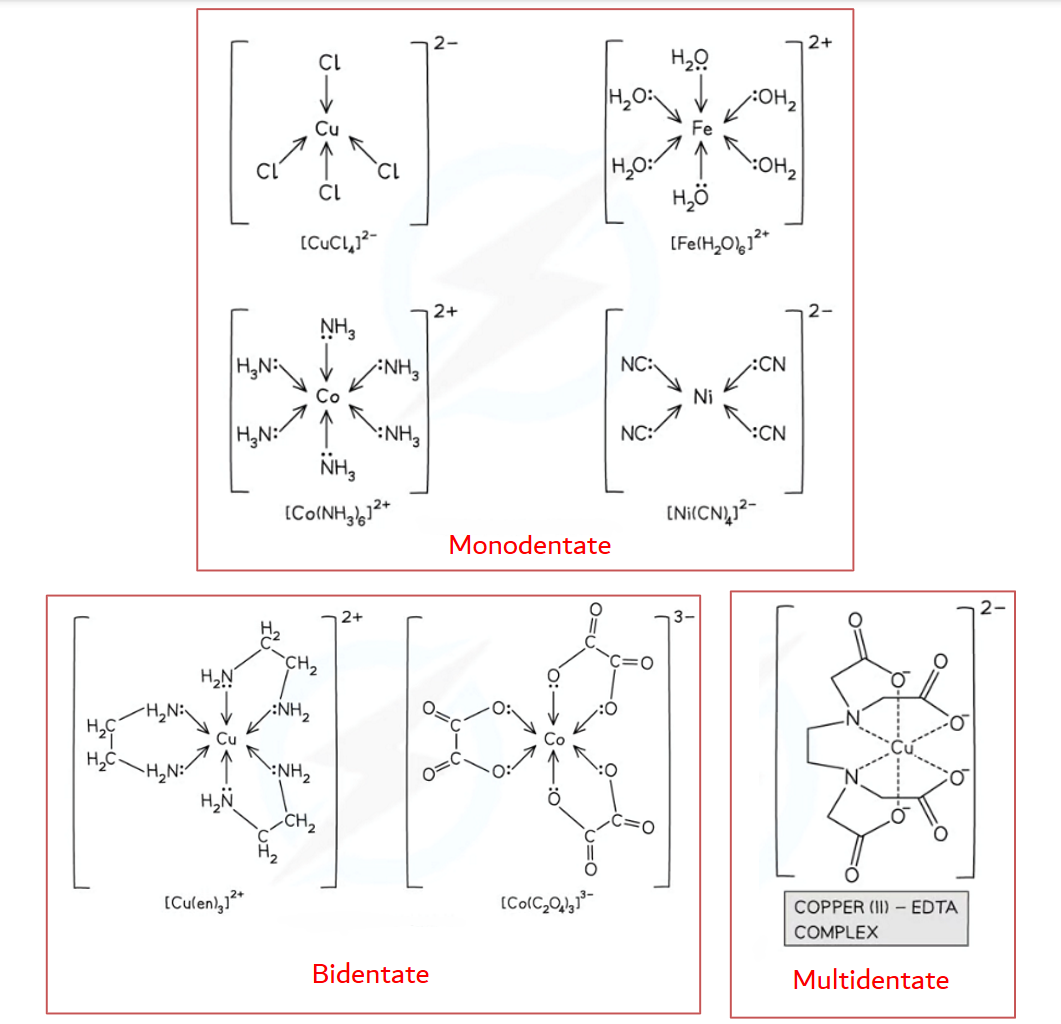
What are monodentate, bidentate and multidentate ligands?
Monodentate- can only form one dative covalent bond to the central metal ion (only contains one atom with a lone pair), eg. water, ammonia, chloride ions, and cyanide ions
Bidentate- can form two dative bonds to the central metal ion (contains two atoms with a lone pair), eg. 1.2-diaminoethane (‘en’), ethandioate ions (‘ox’)
Multidentate- can form more than two dative bonds to the central metal ion (contains more than two atoms with a lone pair), eg. EDTA4-(hexadentate)
How and why can ammonia and water ligands exchange?
Ammonia and water are similar in size and are both uncharged, so water can be substituted for ammonia without changing the coordination number:
Eg. [Co(H2O)6]2+ (aq) + 6NH3 (aq) → [Co(NH3)6 ]2+ (aq) + 6H2O (l) - The coordination number of cobalt stays at 6
In some cases, this substitution is incomplete, so the complex ion contains both water and ammonia ligands:
Eg. [Cu(H2O)6]2+ (aq) + 4NH3 (aq) → [Cu(H2O)2(NH3)4]2+ (aq) + 4H2O (l) - The coordination number of copper stays at 6
How do chloride ligands substitute for water ligands in complex ions?
Chloride ions are larger than water, and are charged, so only 4 of them can fit around the central metal ion, rather than 6
This means that in a chloride ligand substitution the coordination number changes from 6 to 4
Eg. [Cu(H2O)6]2+ (aq) + 4Cl- (aq) → [CuCl4 ]2- (aq) + 6H2O (l)
Eg. [Co(H2O)6]2+ (aq) + 4Cl- (aq) → [CoCl4 ]2- (aq) + 6H2O (l)
Eg. [Fe(H2O)6]3+ (aq) + 4Cl- (aq) → [FeCl4 ]- (aq) + 6H2O (l)
![<ul><li><p><strong>Chloride ions are larger than water, and are charged</strong>, so <strong>only 4 of them can fit </strong>around the central metal ion, rather than <strong>6</strong></p></li><li><p>This means that in a chloride ligand substitution the <strong>coordination number changes from 6 to 4</strong></p></li><li><p>Eg.<strong> [Cu(H<sub>2</sub>O)<sub>6</sub>]<sup>2+</sup></strong><sup> </sup>(aq) + 4Cl<sup>- </sup>(aq) → <strong>[CuCl<sub>4 </sub>]<sup>2-</sup></strong> (aq) + 6H<sub>2</sub>O<sup> </sup>(l)</p></li><li><p>Eg. <strong>[Co(H<sub>2</sub>O)<sub>6</sub>]<sup>2+ </sup></strong>(aq) + 4Cl<sup>- </sup>(aq) → <strong>[CoCl<sub>4 </sub>]<sup>2-</sup></strong> (aq) + 6H<sub>2</sub>O<sup> </sup>(l)</p></li><li><p>Eg. <strong>[Fe(H<sub>2</sub>O)<sub>6</sub>]<sup>3+ </sup></strong>(aq) + 4Cl<sup>- </sup>(aq) → <strong>[FeCl<sub>4 </sub>]<sup>-</sup></strong> (aq) + 6H<sub>2</sub>O<sup> </sup>(l)</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/3b079d64-fa1b-4d9a-bafd-bef8e2c14f22.png)
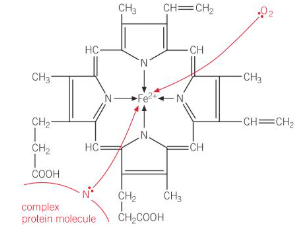
What is the haem complex?
Haemoglobin in red blood cells is made up of a haem complex (Fe (II) + a tetradentate ligand) coordinatively bonded to the nitrogen from a globin protein
This leaves the iron with a sixth space for a ligand to bond, allowing it to accept an oxygen molecule to be carried around the body
Oxygen is not a very good ligand so this dative bond is weak, allowing oxygen to be easily given up to cells
Why is carbon monoxide toxic?
Carbon monoxide is toxic because it is a better ligand than oxygen and binds strongly and irreversibly to the iron(II) in haem complexes, preventing oxygen from being carried to the cells
This is carbon monoxide poisoning, where cells don’t receive enough oxygen for respiration
What is the chelate effect?
The chelate effect is the substitution of monodentate ligands with bidentate and multidentate ligands
This reaction is energetically favourable, as ΔGꝋ is negative
ΔGꝋ = ΔHꝋ – TΔSꝋ
ΔHꝋ, the enthalpy change, is near zero for ligand substitutions because the bonds broken are very similar to those made
ΔSꝋ, the entropy change, is always positive because the reaction produces more particles, as multiple monodentate ligands are displaced
This means TΔSꝋ > ΔHꝋ, so ΔGꝋ is negative and the reaction is favourable
When do complex ions have octahedral shapes?
Octahedral complexes are formed when the central metal ion forms six coordinate bonds
This is possible with:
Six small, monodentate ligands (eg. water, ammonia and hydroxide ions)
Three bidentate ligands
One hexadentate ligand
Other combinations with a coordination number of 6
When do complex ions have tetrahedral shapes?
Tetrahedral complexes are formed when the central metal ion forms four coordinate bonds
This is normally the case for larger ligands like chloride ions
When do complex ions have square planar shapes?
Square planar complexes are sometimes formed when the central metal ion forms four coordinate bonds
This is normally the case for when the central metal ion is platinum or nickel, or when cyanide ion ligands are present
When do complex ions have linear shapes?
Linear complexes are formed when the central metal ion forms two coordinate bonds
The most common examples are [Cu(NH₃)₂]⁺ and [Ag(NH₃)₂]⁺, which is Tollen’s reagent
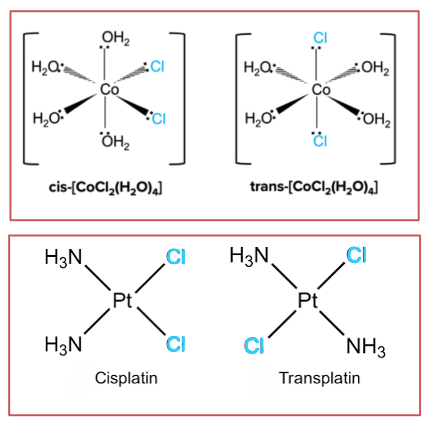
When do complex ions show geometrical isomerism?
Even though transition element complexes do not have a double bond, square planar and octahedral complexes with a pair of ligands different from the rest exhibit cis-trans isomerism
If the two ‘different’ ligands are next to each other, it is the cis isomer
If they are opposite each other it is the trans isomer
Eg. Cis-platin is an anti-cancer drug with a square planar shape
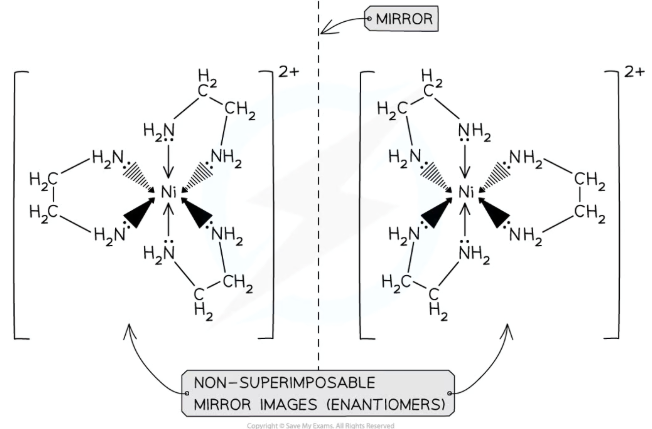
When do complex ions show optical isomerism?
Octahedral complexes containing at least two bidentate ligands can show optical isomerism
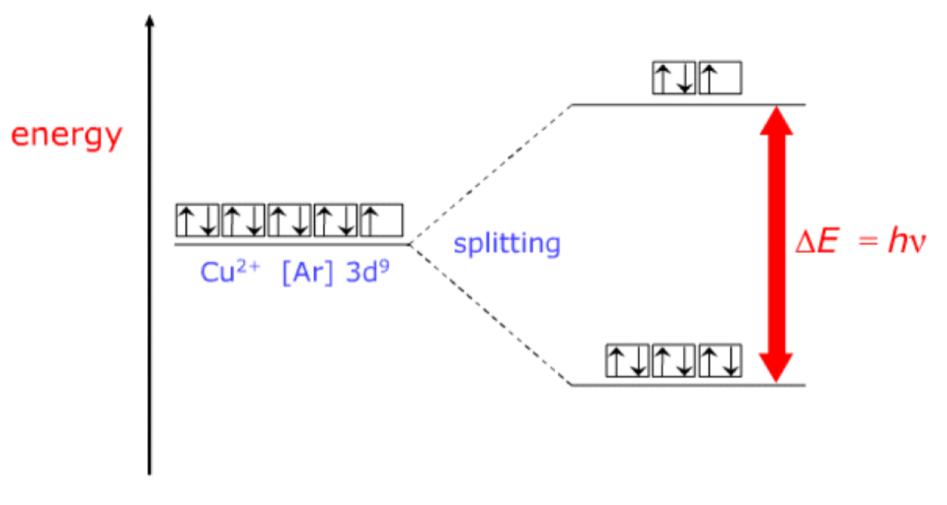
Why are transition metal complexes coloured?
In transition metal complexes, the 3d orbitals exist in two different energy levels
When light is shined on a transition metal complex, an electron from a lower energy d orbital absorbs the energy, ΔE, and enters a higher energy d orbital (this is possible because transition metal ions all have incomplete d subshells)
This electron goes from a ground state to an excited state (higher energy)
The amount of energy, ΔE, needed to excite this electron corresponds to a frequency (colour) of light, according to the equation ΔE = h x v
v = the frequency of light absorbed
h = Planck’s constant
ΔE is dependent on the oxidation state and coordination number of the metal, and the ligands present, so different transition metal compounds absorb different amounts of energy, so they absorb different frequencies and reflect all others (the colours that we actually see)
What is the colour of a transition metal complex dependent on?
The amount of energy, ΔE, needed to excite an electron from a ground state into an excited state is dependent on:
The type of ligand(s)
The coordination number
The oxidation state of the metal ion
ΔE determines the frequencies absorbed according to the Planck’s constant formula, and all the reflected frequencies make up the colours we actually see
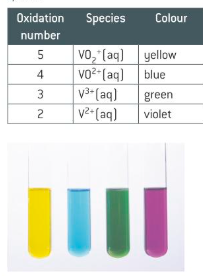
What colours are the different oxidation states of vanadium? How are these formed?
Vanadate (V) ions, VO2+, are reduced by zinc ions in acidic solution to VO2+, V3+, and V2+
What influences the reduction potential of a transition metal ion?
The tendency of a transition metal ion to be reduced is influenced by:
The pH- hydrogen ions are often involved in reduction equations for transition metal ions so low pHs increase the reduction potential
The ligand attached- water is a weaker ligand than ammonia, so it is easier to reduce a transition metal complex with water as the ligand (metal aqua ions)
How does Tollen’s reagent work?
Tollen’s reagent is a colourless solution of [Ag(NH3)2]+ ions, which get reduced to solid silver, Ag, forming a silver mirror with a positive test
This only occurs for aldehydes because they are reducing agents, whereas ketones are not
What are heterogenous catalysts and how do they work?
Heterogenous catalysts are in a different phase from the reactants, and the reaction occurs at active sites on their surface
Since transition metals are expensive precious metals, they are usually only coated onto a support medium with a very high surface area, to increase efficiency while minimising the cost
Eg. Metals are spread over a honeycomb structure in catalytic converters
What are two examples of the use of transition metals as heterogenous catalysts? (Include the equations)
V2O5 is used as a catalyst in the production of sulfuric acid by the Contact process:
Reaction- SO2 (g) + V2O5 (s) → V2O4 + SO3 (g)
Regeneration of catalyst- ½ O2 (g) + V2O4 (s) → V2O5 (s)
Overall equation- 2SO2 (g) + O2 (g) ⇌ 2SO3(g)
The sulfur trioxide produced goes through further reactions to form sulfuric acid
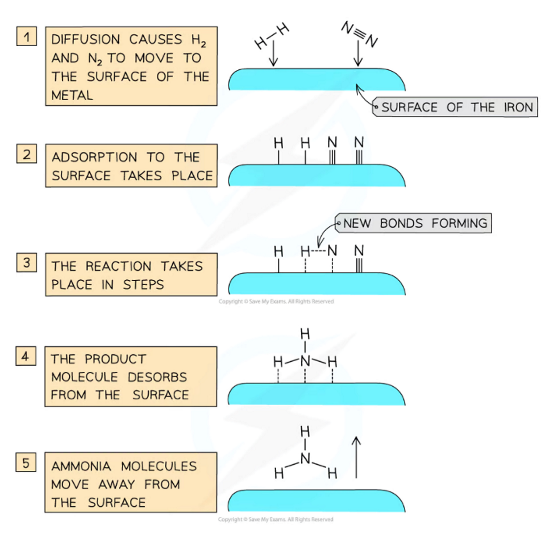
Fe is used as a catalyst in the production of ammonia by the Haber process:
N2 (g) + 3H2 (g) ⇌ 2NH3 (g)
Iron pellets are used as a catalyst to achieve a high surface area
This provides active sites for N2 and H2 to adsorb to, breaking and forming bonds to produce ammonia (in the diagram)
What are the limitations of heterogenous catalysts?
Though catalysts are not used up in reactions, they don’t last forever
This is because impurities build up, bonding irreversibly and blocking the active sites
This ‘poisons’ the catalyst, reducing its efficiency
What are homogenous catalysts and how do they work? Why are transition metals good homogenous catalysts?
Homogenous catalysts are in the same phase as the reactants, and the reaction proceeds through an intermediate species
Transition metals can be used as homogenous catalysts due to their ability to form ions with more than one stable oxidation state, allowing them to act as both oxidising and reducing agents
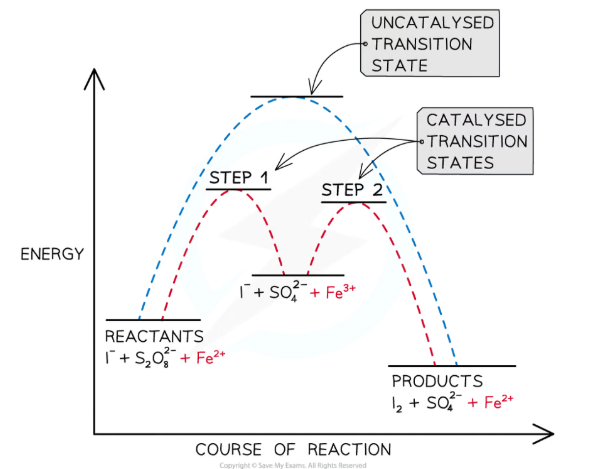
What is an example of the use of a transition metal as a homogenous catalyst?
Fe2+ ions catalyse the reaction between iodide ions, I-, and peroxodisulfate ions, S2O82- :
S2O82- + 2I- → I2 + 2SO42-
This reaction is slow because the ions are oppositely charged, so make few successful collisions- the addition of Fe2+ ions provides an alternative reaction pathway with a lower activation energy, increasing the rate:
S2O82- + 2Fe2+ → 2SO42- + 2Fe3+
2I- + 2Fe3+ → I2 + 2Fe2+
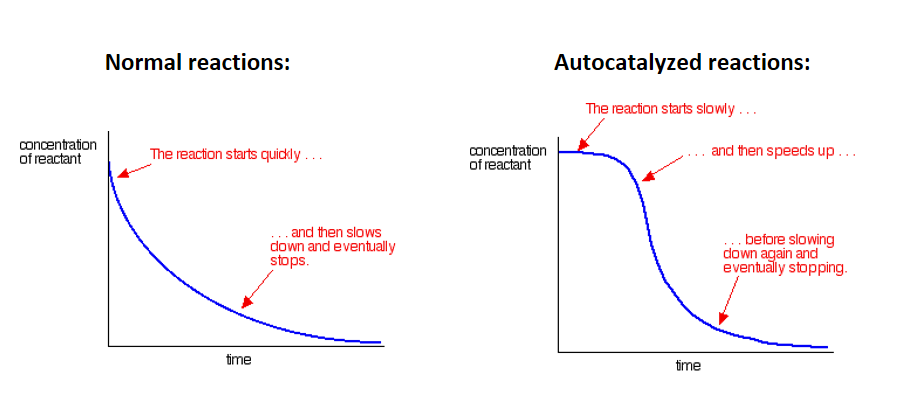
What is autocatalysis and what is an example of it?
Autocatalysis occurs when the product of a reaction acts as a catalyst for it
In the reaction between manganate(VII) ions, MnO4-, and oxalate (ethandioate) ions, C2O42-, the Mn2+ ions produced act as a catalyst:
Half equations- C2O42- → 2CO2 + 2e- and MnO4- + 5e- + 8H+ → 2Mn2+ + 4H2O
Overall equation- 5C2O42- + 2MnO4- + 16H+ → 10CO2 + 8H2O
This reaction is slow because the ions are oppositely charged, however, the production of Mn2+ ions provides an alternative reaction pathway with a lower activation energy, increasing the rate:
4Mn2+ + MnO4- + 8H+ → 5Mn3+ + 4H2O
2Mn3+ + C2O42- → 2CO2 + 2Mn2+
Describe the redox titration using potassium manganate, including the colour change at the end point
In the burette- deep purple potassium manganate, MnO4-
In the conical flask- colourless solution + sulfuric acid (to provide H+ ions for the reduction of MnO4-)
During the titration, the purple MnO4- ions in the burette are reduced to pink Mn2+ ions in the flask, so the mixture in the conical flask will turn from colourless to pink