option-b-biochemistry-flashcards
1/75
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
76 Terms
Metabolism
The sum of chemical reactions occurring in a living organism. Take place in highly controlled aqueous environments (e.g. cytoplasm).
Anabolism
The metabolic pathways of synthesis. Anabolism is an endothermic process (small molecules —> complex molecules).
Catabolism
The metabolic pathways of breakdown. Catabolism is an exothermic process (complex molecules —> small molecules).
Biopolymers (Condensation + Hydrolysis reactions)
Biopolymers are condensation polymers b/c their synthesis involves the loss of an H2O molecule when 2 monomers react. This is called a condensation reaction.
Hydrolysis reactions break down biopolymers in which a polymer breaks up into separate monomers. This requires an H2O molecule for each covalent bond broken.
Photosynthesis vs. respiration
Photosynthesis—the synthesis of energy-rich molecules from CO2 and H2O using light energy
6CO2 (g) + 6H2O (g) —(light energy)-> C6H12O6 + 6O2 (g)
Respiration—the process of chemical breakdown of energy-rich molecules in cells to provide energy
C6H12O6 + 6O2 —> 6CO2 + 6H2O
Aerobic vs. Anaerobic respiration
Aerobic—involves the breakdown of glucose in the presence of molecular oxygen to form CO2 and H2O
C6H12O6 + 6O2 —> 6CO2 + 6H2O
Anaerobic—involves the breakdown of glucose without the presence of molecular oxygen (in humans, lactic acid, 2-hydropropanoic acid, is formed; in plants and yeast, ethanol is formed)
C6H12O6 —> 2CO2 + 2C2H5OH
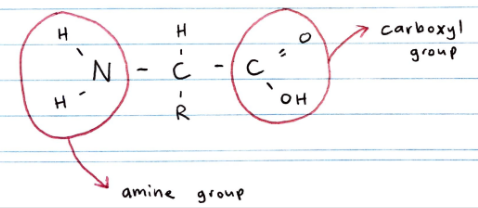
Structure of amino acids
Proteins are polymers of 2-amino acids
Called 2-amino acids b/c the amine group is attracted to carbon 2 (the carbon atom in the COOH group is carbon 1)
Zwitterion
Amino acids are amphoteric due to their acidic carboxyl group and basic amine group. The R groups can also provide some acid-base activity
In aqueous solution and crystalline form, amino acids exist as zwitterions: neutral molecules that have both + and - charges
At the isoelectric point (IEP), an amino acid is electrically neutral
IEP of amino acids
Each amino acid has a unique IEP which depends on the properties of the R group. At the IEP, an amino acid is electrically neutral
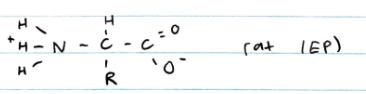
Amino acids and condensation reaction
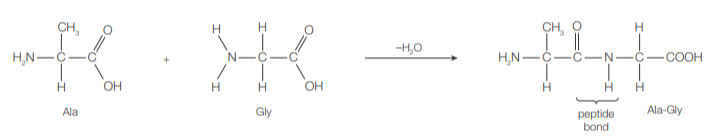
During protein synthesis in cells, amino acids condense (in the presence of enzymes) through the formation of the amide link (–CONH–), or peptide bond, to form a polypeptide chain, which then folds to form a biologically active protein.
Peptide bond forms between the carboxyl group of one AA (C-terminal) and the amine group of another (N-terminal)
Protein structure
Primary Structure
Number and sequence of amino acid (covalent bonding)
Secondary Structure
Folding of a polypeptide chain due to H-bonds between the -C=O group of one amino acid and the -NH group of another
This folding usually results in α-helices (e.g. keratin) or β-pleated sheets (fibroin—spider silk)
Tertiary Structure
Folding of a polypeptide chain due to interactions between R-groups of the AAs
e.g. hydrophobic interactions, H-bonding, ionic bonding, disulfide bridges (covalent)
The resulting 3D shape is the conformation of the protein
Quaternary Structure
Several polypeptide chains combine to form a protein
These polypeptide chains associate via similar forces to those found in the tertiary structure (e.g. disulfide bridges, ionic bonding)
e.g. collagen and hemoglobin
Fibrous vs. Globular proteins
Fibrous
Elongated molecules with a dominant secondary structure
They are physically strong, insoluble in water, and have structural/support functions (e.g. keratin/hair and collagen/skin)
Globular
Compact spherical molecules with a dominant tertiary structure
They are soluble in water and play roles in metabolism (e.g. enzymes, receptors)
e.g. hemoglobin and lactase
Enzymes
Globular proteins that are biological catalysts
The tertiary structure of enzymes gives them a specific 3D shape. This determines the conformation of its active site and thus makes the enzyme specific for a certain substrate
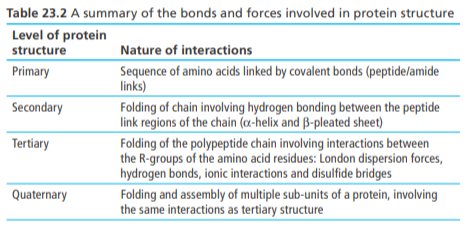
Chromatography (retardation factor, RF)
The components in a mixture have different affinities for the stationary and mobile phase and are hence separated as the mobile phase moves through the stationary phase
This is because different components move up the stationary phase at different speeds and reach different heights
Since AA are colourless, a locating agent like ninhydrin (chemical substance that can detect amines) is sprayed on the stationary phase to aid in their identification
Different AA can be identified by calculating their Rf value. Specific AA have characteristic Rf values, meaning the experimentally desired Rf values can be compared to literature values to identify the AA
Rf = distance traveled by solute (AA)/distance traveled by solvent
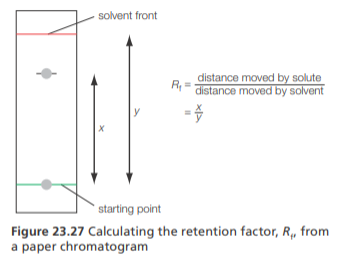
Gel electrophoresis
Can be used to separate a mixture of AA
In gel electrophoresis, an electric current is applied to a gel placed in a buffer solution. This gel contains an AA mixture in pores
If the buffer pH is = to the IEP of an AA, it will remain in the center
If the buffer pH is > the IEP, the AA will exist as an anion and move the anode (+)
If the pH < the IEP, the AA will exist as a cation and move to the cathode (-)
When separation is complete, the AA can be detected by spraying ninhydrin
The rate of movement of the AA ions depends on the no. of charges they have and their molecular mass
Smaller, more highly-charged AA ions move faster (and hence, further)
Types of fatty acids
Fatty acids are carboxylic acids
Fatty acids with no double bonds are saturated, w/ a single double bond are monounsaturated and w/ multiple double bonds are poly-unsaturated
Fats and oils contain a variety of fatty acids and are hence classified by the predominant type of unsaturation present
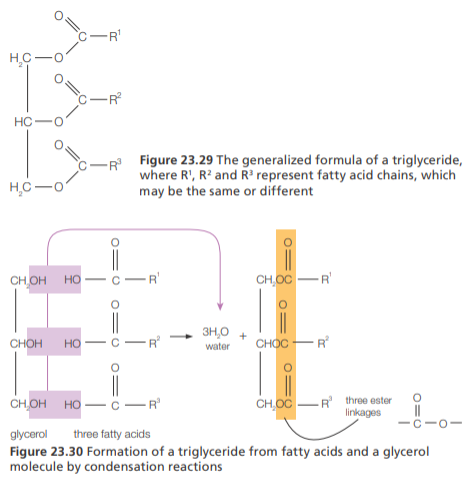
Triglycerides
They are esters formed by condensation reactions between glycerol (C3H8O3) and 3 fatty acids
To form triglycerides, an esterification reaction takes place between the -COOH group of each fatty acid and each -OH group in glycerol, eliminating H2O and forming an ester linkage
Phospholipids
Phospholipids are derivatives of triglycerides
They contain one glycerol, two fatty acids, and one phosphate group
Phospholipids have a polar phosphate “head” and two non-polar fatty acid tails
As a result, phospholipids naturally align themselves to form micelles or phospholipid bilayers
Impact of lipids on health: cholesterol (LDL vs. HDL)
Cholesterol is transported in the bloodstream bound in different lipoproteins (complex particles composed of multiple proteins that transport all fat molecules around the body within the water outside cells): LDL and HDL
High levels of LDL cholesterol are associated w/ increased deposition in arterial walls whereas HDL cholesterol is believed to protect against arterial blockage. This is b/c HDL is thought to transport cholesterol away from the arteries
Low-density lipoproteins (LDLs) carry cholesterol from the liver to the body (hence raising blood cholesterol levels)
High-density lipoproteins (HDLs) carry excess cholesterol back to the liver for disposal (hence lowering blood cholesterol levels)
The main sources of LDL cholesterol are saturated and trans fats
The main source of HDL cholesterol is polyunsaturated fats
Essential fatty acid (omega-3 and omega-6)
Humans can synthesize most fatty acids from carbohydrates, but two (cis)-polyunsaturated fatty acids are considered essential
Alpha-linolenic acid (an omega-3 fatty acid) and linoleic acid (an omega-6 fatty acid) cannot be synthesized by the body
This is because humans lack the enzyme required to introduce double bonds at the required position of the carbon chain
Essential fatty acids are modified by the body to make important lipid-based compounds (such as signaling molecules)
There is evidence to suggest dietary deficiencies of these fatty acids may be linked to impaired brain development (e.g. depression) and altered maintenance of cardiac tissue (e.g. abnormal heart function) – although this evidence is contested
Foods rich in essential fatty acids (omega-3 and omega-6) include fish, leafy vegetables and walnuts
Trans fatty acid
Most fatty acids are cis. Trans fatty acids are often a byproduct of food processing. Trans fats increase LDL levels and lower HDL levels, significantly raising blood cholesterol levels.
Carbohydrates
Have the general formula Cx(H2O)y, where x and y are the same numbers in monosaccharides
Carbohydrates are more oxidized than lipids
Monosaccharides
Monosaccharides are the simplest form of carbohydrates
They contain several hydroxyl groups alongisde either an aldehyde (aldose sugar) or ketone (ketose sugar) funct. group
Monosaccharides can be, for example, triose (C3), pentose (C5) or hexose (C6)
The large no. of -OH groups in monosaccharides make them soluble in polar solvents (e.g. water)
Cyclisation of sugar
In aqueous solutions, monosaccharides form cyclic structures. The carbonyl of the aldehyde/ketone group reacts with an -OH group to form an ether linkage
The cyclic structures of polysaccharides are represented by Haworth projections
In Haworth projections, the carbonyl carbon is given the lowest possible number and this carbonyl group will react with the second-to-last hydroxyl group
Disaccharides
Form by linking two monosaccharides together in a condensation reaction
The result is a glycosidic linkage
Disaccharides are soluble molecules due to their many -OH groups
Polysaccharides
Polysaccharides are formed when more than 2 monosaccharides polymerize by a condensation reaction
Due to their large size, polysaccharides are all insoluble molecules
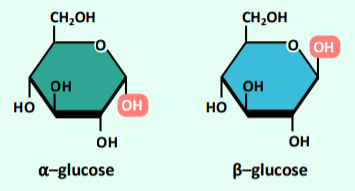
α-glucose vs β-glucose
Glucose monomers exists as two distinct isomers (a-glucose and b-glucose) that differ in structure according to the orienta,on of hydroxyl group (–OH) attached to the 1’–carbon atom.
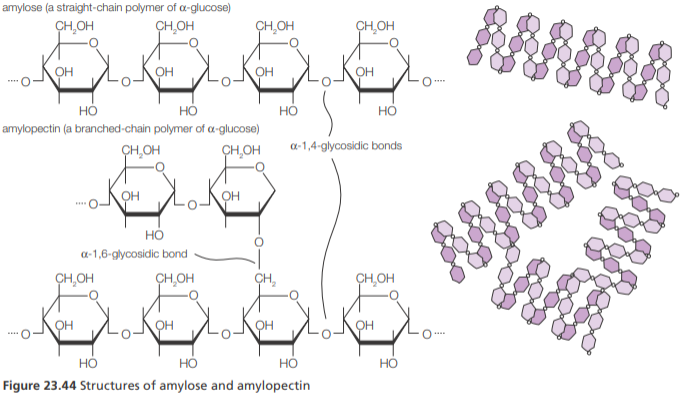
Amylose vs Amylopectin
Amylose is an unbranched polymer of glucose molecules linked by α-1,4 glycosidic bonds.
Amylopectin is a branched polymer of glucose but has many branches arising from α-1,4-glycosidic bonds. Starch contains variable amounts of amylose and amylopectin.
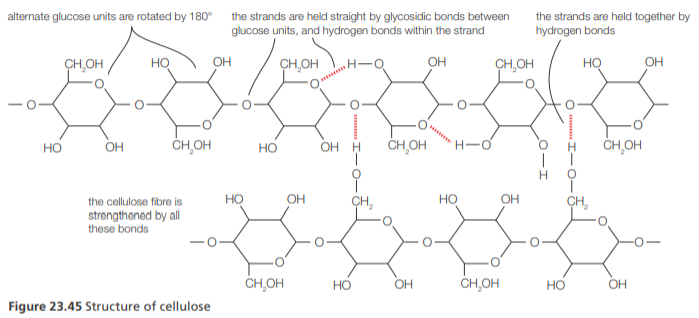
Cellulose
Cellulose is the structural component of plant cell walls.
It is a polysaccharide consisting of glucose units joined by 1, β-4-glycosidic linkages.
Few animals have the cellulose enzyme that can hydrolyze these linkages, allowing them to digest cellulose.
Cellulose molecules are linear because of the orientation of the glucose residues. The molecules are held together by hydrogen bonds between hydroxyl groups and are assembled into cellulose fibres which give tensile strength to plant cell walls.
Dietary fiber
During digestion, enzymes break polysaccharides down into glucose before entering the bloodstream
The human body lacks the enzyme cellulase to break down cellulose
Therefore, cellulose passes through the alimentary canal intact
Cellulose is known as dietary fiber
It stimulates the walls of the digestive tract, producing mucus that allows for the passage of undigested food
Dietary fiber helps reduce conditions such as haemorrhoids and irritable bowel syndrome
Glycosidic bond
A glycosidic bond or glycosidic linkage is a type of ether bond that joins a carbohydrate molecule to another group, which may or may not be another carbohydrate.
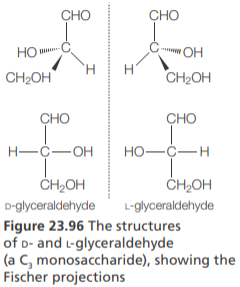
Fischer & Haworth projections
Fischer projections attempt to show 3D structures using a 2D framework of vertical and horizontal bonds.
The main carbon chain is drawn as a vertical line, and bonds to all substituents (atoms or groups of atoms) are drawn as horizontal lines. All vertical lines represent bonds behind the plane of the page, and horizontal lines bonds coming out of the plane toward the viewer
When shown in Fischer projection, if the –OH group on the highest numbered chiral carbon is on the right, the molecule is assigned the label D;
If the –OH group is on the left, it is given the label L. These notations are known as absolute configurations and are often used to describe amino acids and monosaccharides
The amino acids that make up the majority of proteins exist in the L configuration; most naturally monosaccharides exist in the D configuration.
Vitamins as micronutrients
Vitamins are organic compounds which are micronutrients, meaning they are needed in small amounts to maintain health
Vitamins can mostly (except for vitamin D) not be synthesized by the body and must hence be obtained from suitable food sources
Vitamin solubility
Water-soluble
Water-soluble vitamins have polar bonds and the ability to form H-bonds with water
They are transported in the blood (directly) and excesses are excreted
Vitamins B and C are water-soluble
Lipid-soluble
Lipid-soluble vitamins are mostly non-polar molecules with long hydrocarbon chains
They are slower to be absorbed and excesses are stored in adipose tissue where they can produce serious side-effects
Vitamins A, D, E, and K are lipid-soluble
Vitamins A vs C vs D
Vitamin A (retinol)
Lipid-soluble
non-polar hydrocarbon chain and ring influence solubility more than the one -OH group
Required for vision, particularly in low light
Vitamin C (asorbic acid)
Water-soluble
Several -OH groups enable H-bonds to form w/ water
Acts as a cofactor in some enzymatic reactions and important in tissue regeneration
Vitamin D (calciferol)
Lipid-soluble
Hydrogcarbon molecules w/ 3 carbon rings and one -OH group
Can be syntehsized from cholesterol
Stimulates the uptake of Ca2+ ions by cells and important in teeth and bone health
Heat sensitivity of vitamins
Most vitamins are sensitive to heat, but water-soluble vitamins are most sensitive
Heat can cause vitamins to lose their activity given that increased temp. allow vitamins to undergo oxidation reactions at a greater rate, thus changing the compound
Vitamin deficiencies (incl. xerophthalmia and osteoporosis)
When vitamins aren’t available in sufficient quantities in a person’s diet, a vitamin deficiency may result
Vitamin deficiencies can be caused by a lack of food sources for a particular vitamin, over-processing of food, lack of education about a balanced diet, etc.
Vitamin A Deficiency
Vitamin A is needed for healthy eyesight (could lead to xerophthalmia, a disease that causes dry eyes)
Most common in developing countries
Vitamin B Deficiency
Vitamin B deficiency causes a range of diseases like anemia and beriberi
Arise from poor nutrition
Vitamin D Deficiency
Vitamin D is needed for the absorption of micronutrients (e.g. Calcium) and is required for proper bone development; could lead to osteoporosis (bones become weak and brittle)
Deficiency may lead to rickets
Solutions to vitamin deficiencies
Fortification of staple foods (e.g. rice) with vitamins
Producing vitamin supplements
Increased labeling of foods w/ content info
Education regarding balanced diet and nutrition
What are xenobiotics?
Xenobiotics are chemical compounds that are found in a living organism but are foreign to that organism
Ex. incl. drugs (e.g. penicillin), heavy metals (e.g. Pb ions), plastics, and insecticides
Chemicals which are produced in one species and are found in a different species are also considered xenobiotics (e.g. human hormones in fish)
Effect of xenobiotics
Some non-polar xenobiotics may pass across cell membranes into cells and be metabolized (detoxification)
However, if the xenobiotic cannot be broken down, it may build up in cells. This is called bioaccumulation
Antibiotic waste
Pharmaceutically active compounds (e.g. antibiotics and painkillers) may be discharged from hospitals or released by humans in urine into the environment
Sewage treatment plants may break xenobiotics down using bacteria but this process is often incomplete, resulting in pharmaceutical compounds being released into the effluent
Biomagnification
Biomagnification refers to the increase in conc. of a xenobiotic substance in a food chain
Typically, polar and water-soluble compounds tend not to accumulate in organisms since they can be excreted in sweat or urine
However, xenbiotic compounds which are non-polar and can’t be metabolized may accumulate in living organisms and be passed on when one organism feeds on another
Animals at the top of the food chain consume others below them, making accumulated xenobiotics more conc. at each level of a food chain
Biomagnification: DDT
DDT is an insecticide that is fat-soluble and doesn’t undergo metabolic breakdown, thus causing it to bioaccumulate and pass through food chains
It is now banned worldwide due to the effects of its biomagnification
Dioxins (PCCDs, PCBs)
Three well-studied classes of xenobiotics are dioxins, dioxin-like substances, such as polychlorinated dibenzodioxins (PCCDs), and polychlorinated biphenyls (PCBs).
Dioxins are produced as by-products in the manufacture of chlorinated organic compounds and the incineration of plastics.
They are highly carcinogenic, especially chlorinated dioxins, and they can disrupt the endocrine system (hormone action) and lead to cellular and genetic damage.
PCBs contain one to ten chlorine atoms attached to a biphenyl molecule. They are highly stable, with high electrical resistance, and were used as coolants, plasticizers, lubricants, and insulating liquids.
Host-guest chemistry
Host-guest chemistry involves the synthesis of a host molecule which binds non-covalently to a guest molecule to form a supermolecule
H + G ⇌ H—G
The forces within the supermolecule are non-covalent (e.g. ionic bonds, H-bonds, hydrophobic interactions)
Essentially, the host can be seen as analogous to an enzyme, the guest to the substrate, and the supermolecule to an E—S complex
Use of host-guest chemistry to remove xenobiotics
The xenobiotic substrate acts as the guest, and its chemical features determine the synthesis of the host, which is designed to selectively bind to it
This can be used to remove radioactive caesium/cesium ions (guest) from nuclear waste
Biodegradability
Substances that can’t be broken down by natural processes are non-biodegradable
Ex. incl. plastics like PVC
Substances which can undergo bacterial degradation into end products that are formed in nature are biodegradable
Types of biodegradable plastic
Plant-based
This plastic has a high starch content
Starch grains swell and help break up the plastic into smaller pieces, making it more accessible to bacteria
The carbohydrate grains are hydrolyzed by bacteria
Petroleum-based, oxo-biodegradable
Derived from a by-product of the oil industry
Additives are used to act as catalysts for the breakdown of this plastic into microfragments which are eventually broken down by bacteria
Breakdown of oil spills by enzymes
Bioremediation is the breakdown of oil by microorganisms who use it as a food source and oxidize it in respiration (producing CO2 and H2O)
Certain microorganisms have evolved different enzymes that are specific for the degradation of different hydrocarbons in oil
Ultimately, enzymes can be used to help in the breakdown of oil spills and other industrial wastes (e.g. textile industry)
Enzymes in biological detergents
Detergents are water-soluble compounds that are used to dissolve impurities
Biological detergents contain enzymes that are capable of breaking down impurities to make them more soluble and easier to wash away
The use of these enzymes allows for effective cleaning at lower temp. and using less detergent
Green chemistry
Green chemistry is an approach to chemical research and engineering that seeks to minimize the production and release to the environment of hazardous substances
When assessing the “greenness” of a substance used in biochemical research, scientists consider the yield of a reaction, the atom economy (mass of desired prod./mass of reactants), and energy efficiency
Examples of green chemistry
Superficial CO2 can replace toxic solvents used in, for example, making decaffeinated coffee
Plant-based plastics, which are biodegradable, can replace non-biodegradable oil-based plastics
Production of esters for creams traditionally uses H2SO4 at high temp., but can be done using enzymes at room temp.
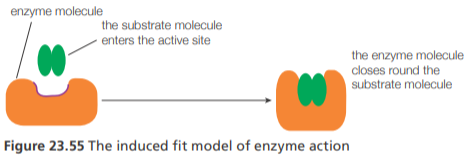
Enzyme activity (induced fit theory)
At the active site of the enzyme, the amino acid side chains of the folded protein are precisely positioned so they favor the formation of the high-energy transition states that the substrate(s) must pass through to be converted to product.
The active site of some enzymes changes shape when the substrate binds. This finding led to the development of the induced-fit model of enzyme activity
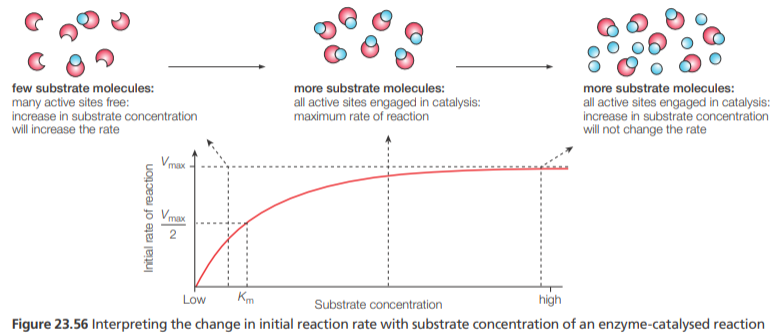
Enzyme kinetics (Michaelis-Menten, Km)
The kinetics of enzyme-controlled reactions (known as Michaelis-Menten kinetics) identifies two important parameters:
Vmax and Km (the Michaelis constant), the concentration of substrate that results in half the maximum rate
The maximum reaction rate for a given concentration of enzyme at a specified temperature and other conditions, e.g. pH, is referred to as Vmax
Low Km— indicates that the enzyme works efficiently even if the concentration of the substrate is low
High Km—indicates that the enzyme requires a high substrate concentration before being relatively active
substrate
A substrate is the medium in which a chemical reaction occurs or the reagent in a process that provides a surface for absorption.
active site
The active site of an enzyme is the binding site for the substrate and a catalytic site which completes the enzyme-catalyzed reaction.
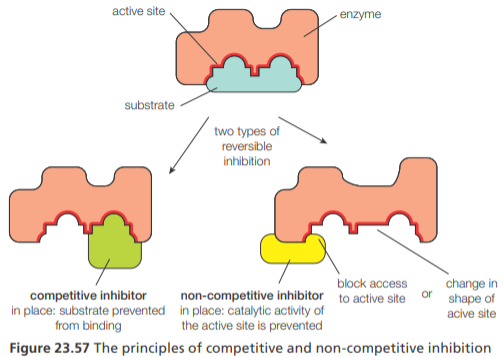
Inhibition
There are two common types of reversible inhibition of enzymes, competitive and non-competitive inhibition. They can be distinguished by their effects on enzymes
Competitive inhibition— involves a molecule binding to the active site of an enzyme and thus preventing the substrate from binding. The inhibitor has a similar structure to the substrate. Competitive inhibition does not affect the value of Vmax but increases Km
Non-competitive inhibition— involves a molecule binding to the enzyme at a site distinct from the active site. This alters the shape of the enzyme, affecting the active site so that it no longer binds the substrate. Noncompetitive inhibition decreases the value of Vmax but does not affect the value of Km
Allosteric site
The product of the last reaction of the metabolic pathway will bind to a site other than the active site of the enzyme that catalyzes the first reaction. This site is called the allosteric site
When it binds to the allosteric site it acts as a non-competitive inhibitor and changes the conformation of the active site
Therefore, it makes the binding of the substrate to the enzyme unlikely
Once the inhibitor is released from the allosteric site, the active site returns to its original conformation and the substrate can bind again
Nucleotides (Incl. adenine, thymine, cytosine, guanine, and uracil)
Nucleotides are the condensation products of a pentose sugar, phosphoric acid, and a nitrogenous base – adenine (A), guanine (G), cytosine (C) and thymine (T) or uracil (U).
Pentose sugar:
In DNA, it’s deoxyribose; in RNA it’s ribose
The difference between these sugars is the groups attached to C-2, deoxyribose lacks an -OH
Phosphate group:
PO43-
Derived from H3PO4
Nitrogenous base:
Purines are larger and contain two fused rings. Adenine and guanine are purines
Pyrimidines are smaller and contain a single ring. Cytosine, Thymine, and Uracil are pyrimidines
Polynucleotides
Polynucleotides form by condensation reactions. It is a combination of nucleotide monomers which are connected to each other through covalent bonds.
Histones
Chromosomes consist of DNA bound to specialized, highly basic proteins which help fold the DNA into a compact form
These basic proteins are known as histones and they have a high content of amino acid residues with basic positively charged R-groups
Triplet code
The sequence of bases in DNA determines the primary structure of proteins synthesized by the cell using a triplet code, known as the genetic code, which is universal.
Codon
The base sequence of an mRNA molecule encodes the production of a polypeptide
The mRNA sequence is read by the ribosome in triplets of bases called codons
Each codon codes for one amino acid with a polypeptide chain
The order of the codons in an mRNA sequence determines the order of amino acids in a polypeptide chain
DNA
DNA is a double helix of two polynucleotide strands held together by hydrogen bonds.
RNA
RNA is usually a single polynucleotide chain that contains uracil in place of thymine and a sugar ribose in place of deoxyribose.
Human genome
The human genome contains approximately 3 × 109 nucleotide base pairs divided among 23 pairs of chromosomes (in humans). Only a small percentage of the DNA codes for proteins and RNA
The remainder is a mixture of ‘junk’ and sections that regulate the decoding, or expression, of the genes themselves
The functional regions of DNA are known as genes
Individual humans differ from each other by an average of 1 nucleotide pair in every 1000; this variation is the basis for our genetic individuality and is the basis for identifying individuals by DNA ‘fingerprinting’
Porphyrins
Poryphrins are chelates of metals w/ large, nitrogen-containing macrocyclic ligands
Poryphryins contain a planar ring structure composed of four linked heterocyclic rings
The non-bonding pairs of e- on the nitrogen atoms of this ring allow it to form coordinate covalent bonds w/ a central metal ion, thus making it a ligand
Different porphyrins (e.g. heme and chlorophyll) contain different metals
Heme
Heme is the prosthetic group of hemoglobin and myoglobin and contains an iron ion (Fe2+) —> also in cytochrome
Heme appears red in color
Hemoglobin and myoglobin
Hemoglobin carries oxygen in the blood and contains four heme groups and four polypeptide chains (quaternary structure)
Myoglobin stores oxygen in cells and contains one heme group and one polypeptide chain
Both molecules reversibly bind w/ oxygen (O2) which forms a weak bond w/ the Fe2+ ion
When this happens, oxyhemoglobin and oxymyoglobin are produced respectively
Due to the no. of heme groups, hemoglobin can bind to 4 O2 molecules whereas myoglobin can only bind to one
Hb + 4O2 ⇌ Hb—(O2)4
Mb + O2 ⇌ Mb—O2
Fetal hemoglobin and myoglobin
Fetal hemoglobin is a different form of hemoglobin only present in the blood of the developing fetus
It has a higher affinity for oxygen than maternal hemoglobin (which replaces it after birth)
This adaptation allows the efficient transfer of oxygen from the mother’s blood to the fetal blood in the placenta
Myoglobin also has an oxygen dissociation curve to the left of that of hemoglobin, which means it has a greater affinity for oxygen and can accept oxygen from hemoglobin for storage (in the striated muscles)
Cytochromes
Cytochromes are a group of protein molecules that contain the heme prosthetic group
They are found in membranes and are responsible for e- transport during the redox reactions of aerobic respiration and photosynthesis
In cytochrome, the iron of the heme group interconverts its ox. state between +2 and +3 as the cytochrome undergoes redox change (accepts e- (reduction) and passes them on (oxidation))
Anthocyanins
Anthocyanins are a group of water-soluble, aromatic pigments and are typically pink, red, or blue in color (absorb blue/green light)
They have a three-ring C6C3C6 structure and conjugated C=C double bonds
Their water-solubility is due to their polar -OH group, which allow them to form H-bonds
Cooperative binding
The binding of Hb to O2 is cooperative, meaning that the ability to bind O2 increases by the initial binding of O2to a heme group
This cooperative effect is due to subtle conformational changes to Hb when O2 binds to one heme group, making other heme group shave a greater affinity for O2
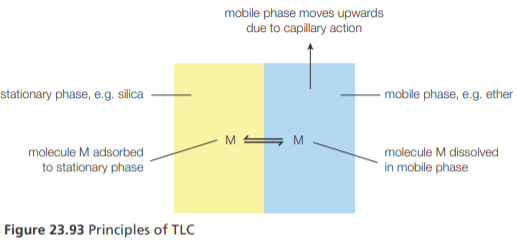
Thin-layer chromatography
Thin-layer chromatography (TLC) uses a stationary phase of silica or alumina particles bonded to a thin layer of glass or plastic
TLC separates a mixture of pigments based on how strongly they are adsorbed on the stationary phase and dissolved in the mobile phase (a liquid or mixture of liquids)
This equilibrium is known as partitioning. The greater the affinity of the pigment for the stationary phase, the more slowly it moves along the surface of the TLC plate.
TLC is faster, more sensitive, and has better resolution than paper chromatography
Starch
Starch is the food storage material of plants. It is a polysaccharide, which occurs in two forms: amylose and amylopectin. Both are condensation polymers of glucose and are poorly soluble in water.
It is a polymer of α- glucose
Form compact spiral structures
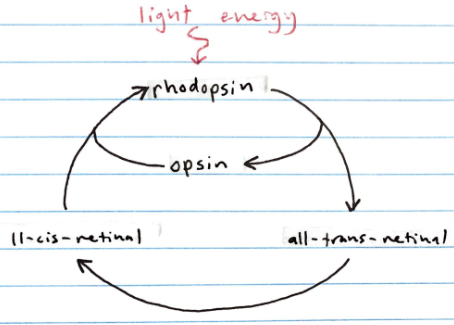
The visual cycle (incl. rhodopsin, opsin, cis-retinal, trans-retinal)
The retina of the eye contains two types of light-sensitive cells: rods and cones
Rods are stimulated by low intensity light (no color vision)
Rhodopsin is a conjugated protein found in the rods which is made of a protein called opsin that’s bound to 11-cis-retinal (drerived from vitamin A)
When rhodopsin is exposed to light, 11-cis-retinal is isomerized to all-trans-retinal
This isomerization results in all-trans-retinal dissociating from the opsin, which triggers a nerve impulse
Afterward, enzymes convert all-trans-retinal back into 11-cis-retinal so it can bind to a new opsin protein, thus regenerating rhodopsin