Combustion of alkanes as fuels
1/4
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
5 Terms
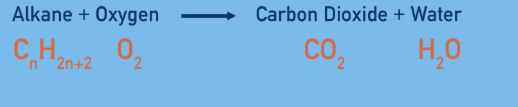
What is complete combustion
Shorter chain alkanes are more likely to undergo complete combustion
What is incomplete combustion
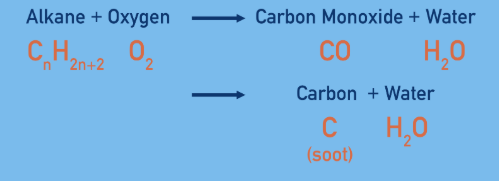
What are the consequences of incomplete combustion
Combustion of sulfur containing fuels causes sulfur dioxide (SO2) to be released into the atmosphere, this can lead to acid rain
High temperature and pressures inside internal combustion engines causes nitrogen and oxygen to react together, forming nitrogen oxides (NOx), that can lead to acid rain.
How can flue gases be removed
We can remove sulfur dioxide by reacting with calcium oxide or calcium carbonate.
This is because sulfur dioxide is acidic and calcium oxide is basic. The balanced equation for the process is:
CaO +SO2 → CaSO3
CaCo3 is a solid so its easier to collect
What can be used to remove nitrous oxides from fuel
Catalytic converters can remove unburned hydrocarbons and nitrous oxides from fuel such as platinum or rhodium.