Uncertainty in measurements: 4.3.1 Chemical measurements, conservation of mass and the quantitive interpretation of chemical equations: AQA Q&A: GCSE (9:1)
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
12 Terms
23.5°C
A student reads a value of 23.0 degrees from a thermometer. The thermometer has an uncertainty of ₊⁻0.5°C. What is the maximum possible temperature for this reading?
Thermometer B has a higher resolution
A student has a choice of thermometers. Thermometer A reads to the nearest 0.5°C, and thermometer B reads to 2 decimal places. Which statement can be deduced from this information?
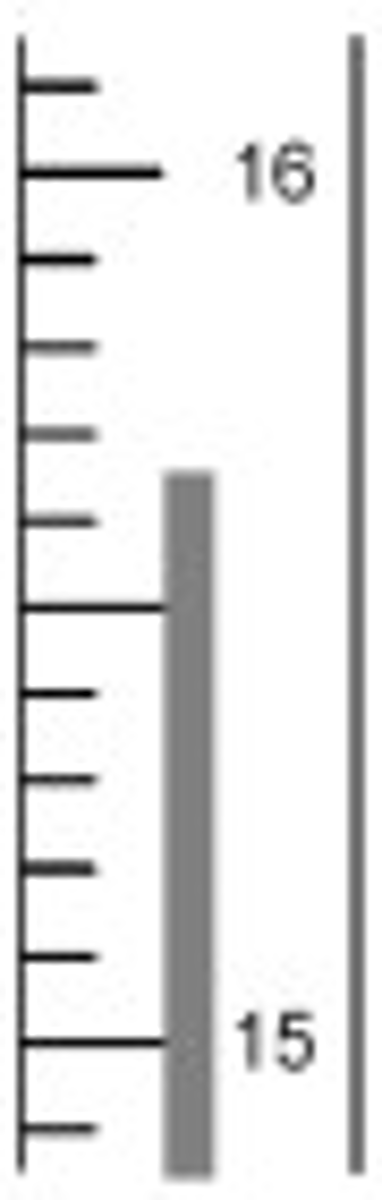
15.65
What is the temperature reading on this thermometer?
24.25
In an experiment the following four volume measurements were taken:
24.5 cm³, 24.0 cm³, 23.5 cm³, 25.0 cm³
Calculate the mean for these measurements
1.5 cm³
In an experiment the following four volume measurements were taken:
24.5 cm³, 24.0 cm³, 23.5 cm³, 25.0 cm³
Calculate the range for these measurements
0.75 cm³
In an experiment the following four volume measurements were taken:
24.5 cm³, 24.0 cm³, 23.5 cm³, 25.0 cm³
Calculate the uncertainty for these measurements
3
In an experiment the following four volume measurements were taken:
24.5 cm³, 24.0 cm³, 23.5 cm³, 25.0 cm³
How many significant figures are these values to?
±0.005 g
A balance is used to record the mass of a substance to 2 decimal places. What is the uncertainty value for this balance?
They are not to the same number of decimal places
A student repeats an experiment three times and records the following temperature changes for the same reaction conditions:
14.5 °C 10°C 9.5°C
What is wrong with the way the results have been recorded?
11.3 °C
A student repeats an experiment three times and records the following temperature changes for the same reaction conditions:
14.5 °C 10°C 9.5°C
Calculate the mean for the results, using all the readings, and record your answer to three significant figures
9.8 °C
A student repeats an experiment three times and records the following temperature changes for the same reaction conditions:
14.5 °C 10°C 9.5°C
Calculate the mean for the results, using only results that lie within 1°C of each other. Give your answer to two significant figures
± 2.5 °C (previously written 5 but prob. wrong)
A student repeats an experiment three times and records the following temperature changes for the same reaction conditions:
14.5 °C 10°C 9.5°C
Calculate the uncertainty for these values