Chap 9C - Acid-base equilibria
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
Define and describe indicators
(Def.): Weak acid or base which appears as different colours in solutions with different pH
Usually organic dyestuffs which are weak acids / bases
Describe methyl orange + equation
HIn (aq) + H2O (I) -> H3O+ (aq) + In- (aq)
KIn = [H+][In-] / [HIn], where KIn is a constant at constant temperature
Different indicators have different KIn values
Describe equation in acid, basic and neutral solution
In acidic solutions: POE shifts left -> [HIn] > [In-] -> solution turns red
In alkaline solutions: POE shift right -> [HIn] < [In-] -> solution turns yellow
In neutral solutions: [HIn] = [In-] -> solution turns orange -> KIn = [H+] and pH = pKIn
Describe working range of indicator
Methyl orange does not appear only orange at pH = pKin
Our human eye is not sensitive enough to detect the specific shade of orange when the solution contains exactly equal proportion of both forms
We see orange when the ratio of both forms of the indicator ranges from 10:1 to 1:10
For [HIn] : [In-] = 10 : 1, Ka = [H+] / 10 -> pKa - 1
For [HIn] : [In-] = 1 : 10, Ka = [H+]10 -> pKa + 1
Based on ratios, pH range is pKa +- 1 (working pH range of indicator)
Since pKa of methyl orange is 3.4 -> working range = 3.1-4.4

Describe colour change, pKIn and pH range of these indicators
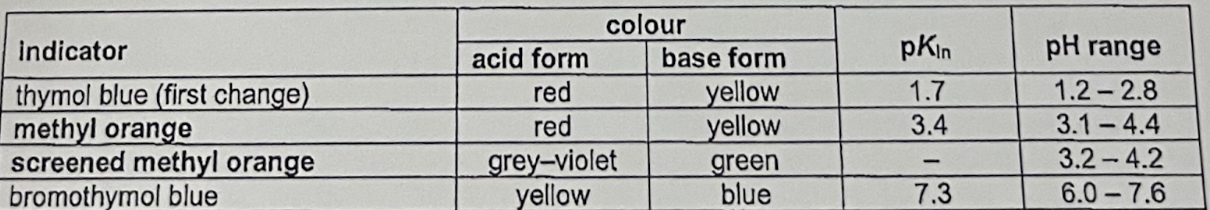

Describe colour change, pKIn and pH range of these indicators

What to take note of when selecting indicator?
The colour observed for an indicator indicates that the pH of a solution is above a certain value or below a certain value -> colour does not directly tell us whether solution is acidic or alkaline
Eg. Yellow for methyl orange implies that the solution has a pH > 4.4
Hence, the indicator working range should lie within the rapid pH change at equivalence point (the vertical part of the titration curve)

Describe indicator used for these type of titration (pH change, indicator)
NOTE: A solution of a weak acid cannot be titrated with a weak base using an indicator to find the endpoint because the pH change is too gradual close to the equivalence point

Describe end VS equivilance point
End point: observed experimentally when the indicator changes color, signaling that the titration should stop
Equivalence point: theoretical point
Define and describe buffer solution
(Def.): A solution that maintains a fairly constant pH when a small amount of acid or base is added to it
Solution must contain a large reservoir of both an acidic component and a basic component that will react with the small amount of base or acid added, respectively
Describe acidic buffer
Solution of weak acid and its salt (salt contains conjugate base of weak acid) (Eg. CH3CO2H)
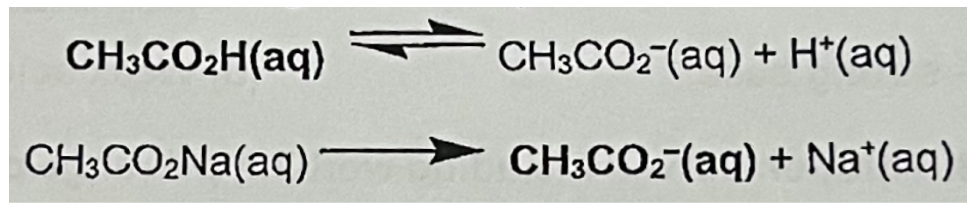
Describe Acidic buffer containing CH3CO2H and CH3CO2Na (include equation and how it can resist change to pH when acid or base added)
CH3CO2Na is fully dissociated in aqueous solution and the CH3CO2- ions produced suppress the dissociation of the weak acid, by Le Chatelier's Principle
The resultant solution, the acidic buffer, contains large reservoirs (high concentrations) of undissociated CH3CO2H molecules (acidic component) and CH3CO2- ions (basic component) which enable the solution to resist changes in pH when:
Acid added to buffer
Large reservoir of ions from the salt remove the added H+ ions -> pH remains constant
CH3CO2- (aq) + H*(aq) → CH3CO2H(aq)
Base added to buffer
Large reservoir of undissociated molecules remove the added OH- ions -> pH remains constant
CH3CO2H(aq) + OH- (aq) → CH3CO2- (aq) + H2O(l)
No reversible sign as a buffer resists changes in pH -> majority of OH- ions removed -> single arrow to show complete reaction
Describe basic buffer and Base buffer containing NH3 and NH4CI
Solution of weak base and its salt (salt contains conjugate acid of weak base) (Eg. NH3)
Eg. Base buffer containing NH3 and NH4CI
NH4C/ is fully dissociated in aqueous solution and the NHa ions produced suppress the dissociation of the weak base, NH3, by Le Chatelier's Principle
Basic buffer, contains high concentrations of both undissociated NH3 molecules (basic component) and NH4+ ions (acidic component) which enable the solution to resist changes in pH when:
Acid added to buffer
Large reservoir of undissociated NH3 molecules remove the added H+ ions
Base added to buffer
Large reservoir of NH4+ ions from the salt NH4Cl remove the added OH- ions