Carbohydrates
1/66
Earn XP
Description and Tags
Exam 3
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
67 Terms
Introduction
Carbohydrates are the most abundant biological molecules
Saccharides (Greek “sugar)
Monosaccharides- the basic carbohydrate unit, “the simple sugars”
Polysaccharides- multiple monosaccharides linked together
Roles of carbohydrates:
Energy Sources
Structural materials
Facilitate protein interaction
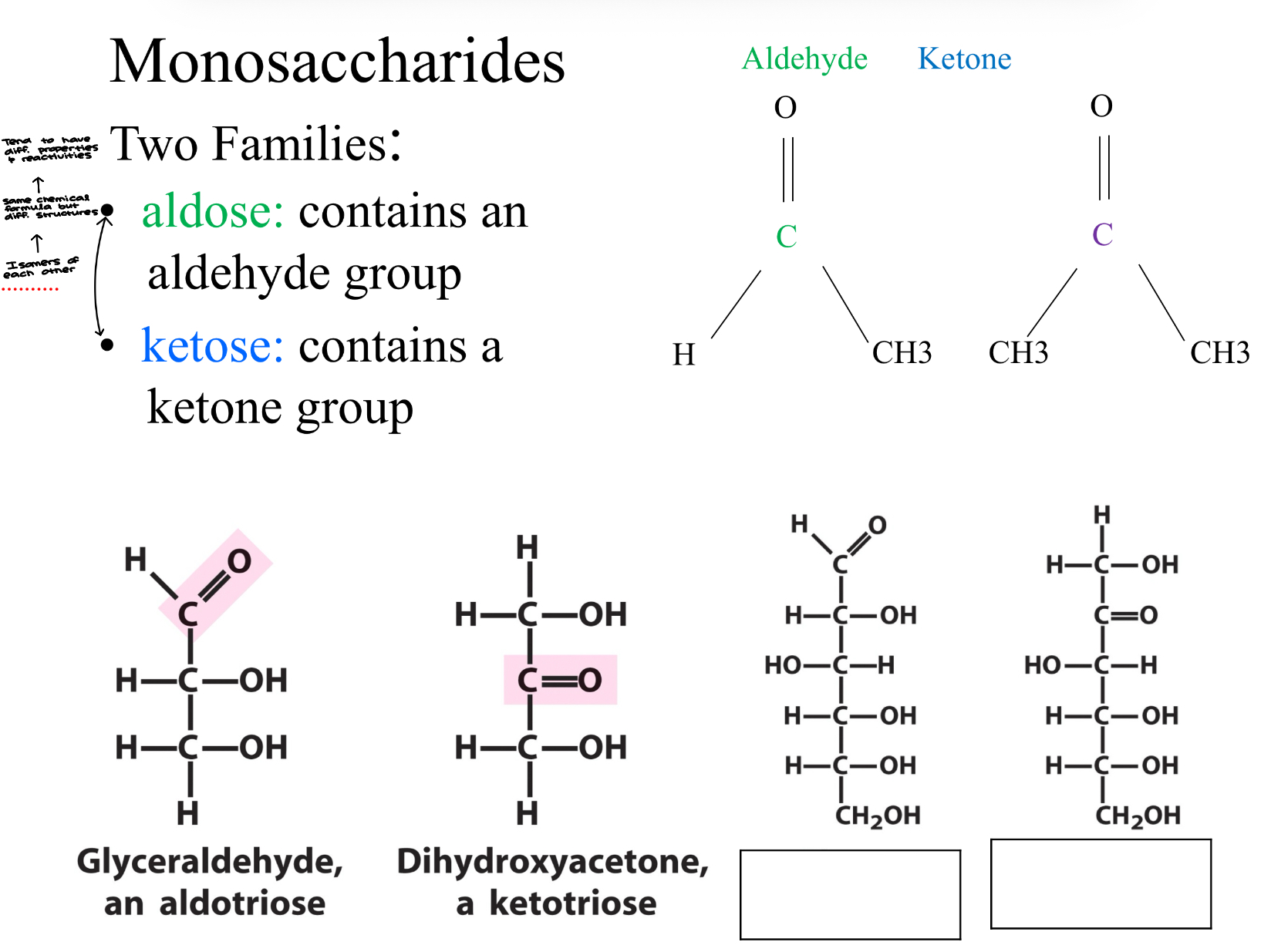
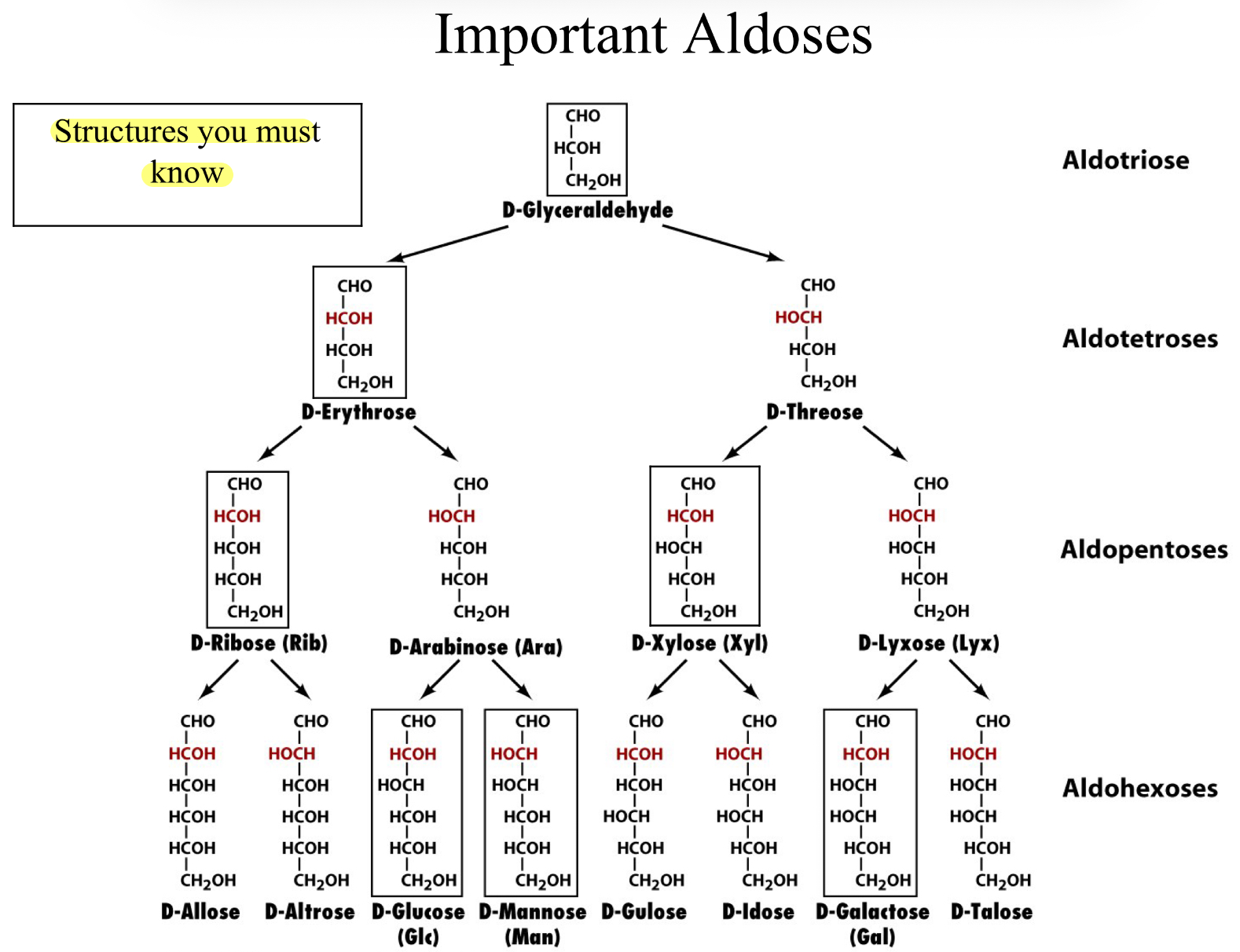
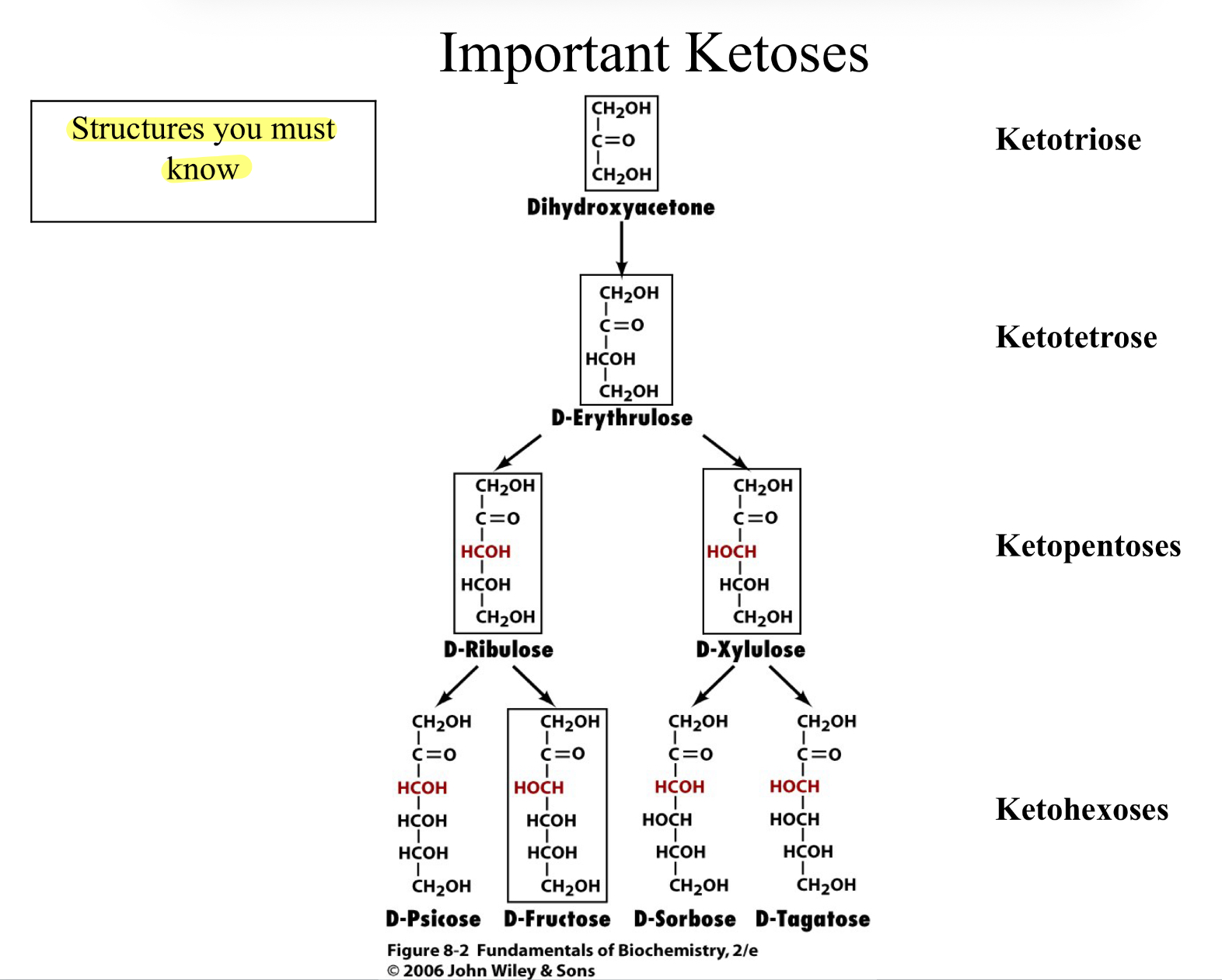
Classification of Monosaccharides- The Simple Sugars
Monosaccharides are classified according to their carbonyl group and the number of carbon atoms
Carbonyl group
If the carbonyl group is an aldehyde then the monosaccharide is an aldose
If the carbonyl group is a ketone, then the monosaccharide is a ketose
Number of Carbons
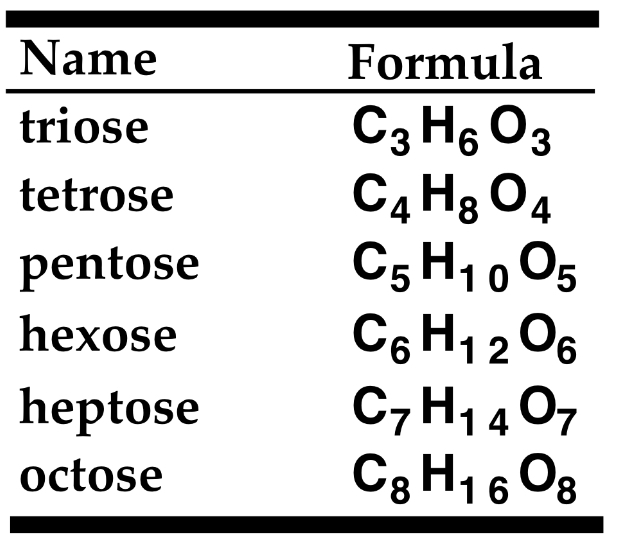
- 3 Carbons- Triose
- 4 Carbons- Tetrose
- 5 Carbons- Pentose
- 6 Carbons- Hexose
- 7 Carbons- Heptose
- 8 Carbons- Octose
Saccharides
They are composed of C, O, H according to the formula (C*H2O)n where n >- 3
Monosaccharides are classified by their number of carbon atoms
Basic carbohydrate units are monosaccharides
Polymers of monosaccharides units are polysaccharides
Triose: C3H6O3
Tetrose: C4H8O4
Pentose: C5H10O5
Hexose: C6H12O6
Heptose: C7H14O7
Octose: C8H16O8
Monosaccharides
Two Families:
Aldose: contains an aldehyde group
Ketose: Contains a ketone group
aldose and ketose are to be isomers of each other, they have the same chemical formula but different structures… they tend to have diff. properties and reactivities
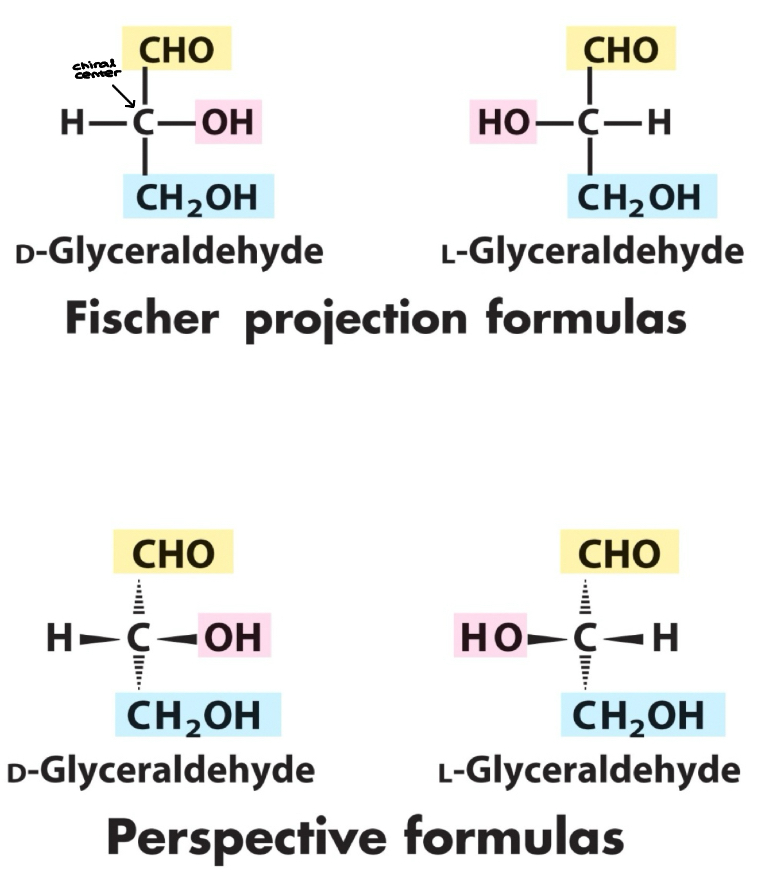
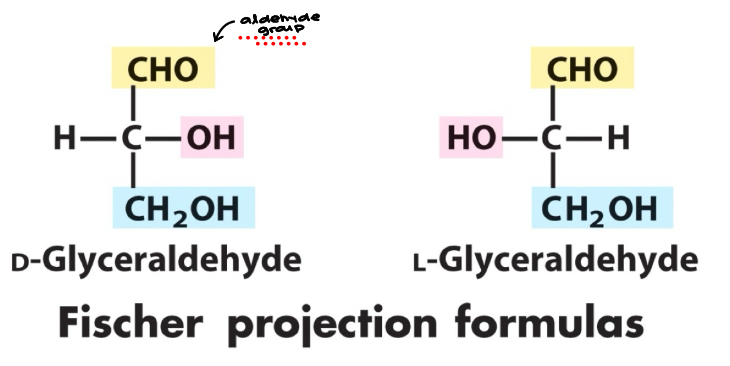
Fischer Projection
Fischer projection: a two dimensional representation for showing the configuration of tetrahedral stereocenters
D & L
According to the conventions proposed by Fischer (Bottom -OH is…)
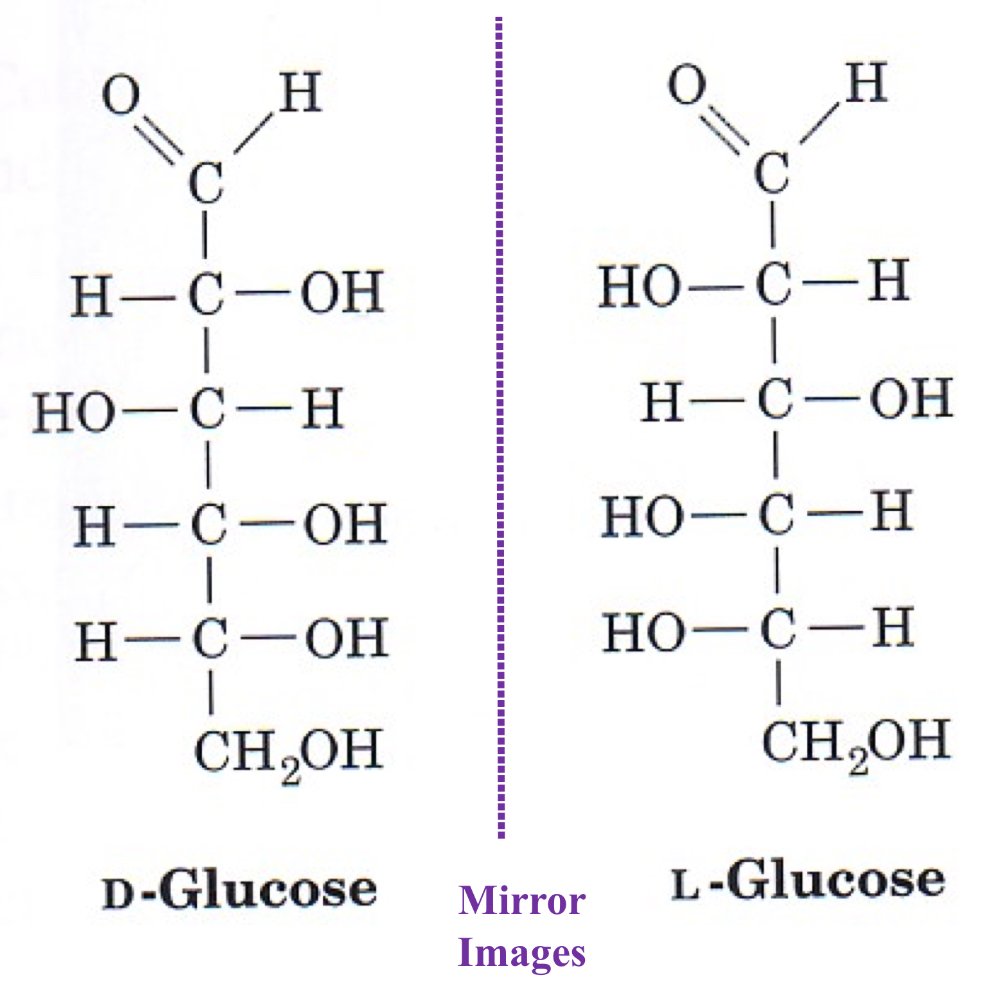
D-monosaccharide: a monosaccharide that, when written as a Fischer projection, has the -OH the right of the chiral carbon
L-monosaccharide: a monosaccharide that, when written as a Fischer projection, has the -OH on the left of the chiral carbon
Monosaccharides 2
Contain one or more chiral carbons
Active isometric forms (meaning they are isomers)
Simple aldose: glyceraldehyde
1 chiral carbon
2 isometric forms
A molecule with n chiral centers can have 2^n stereoisomers
Enantiomers
Stereoisomers that are mirror images
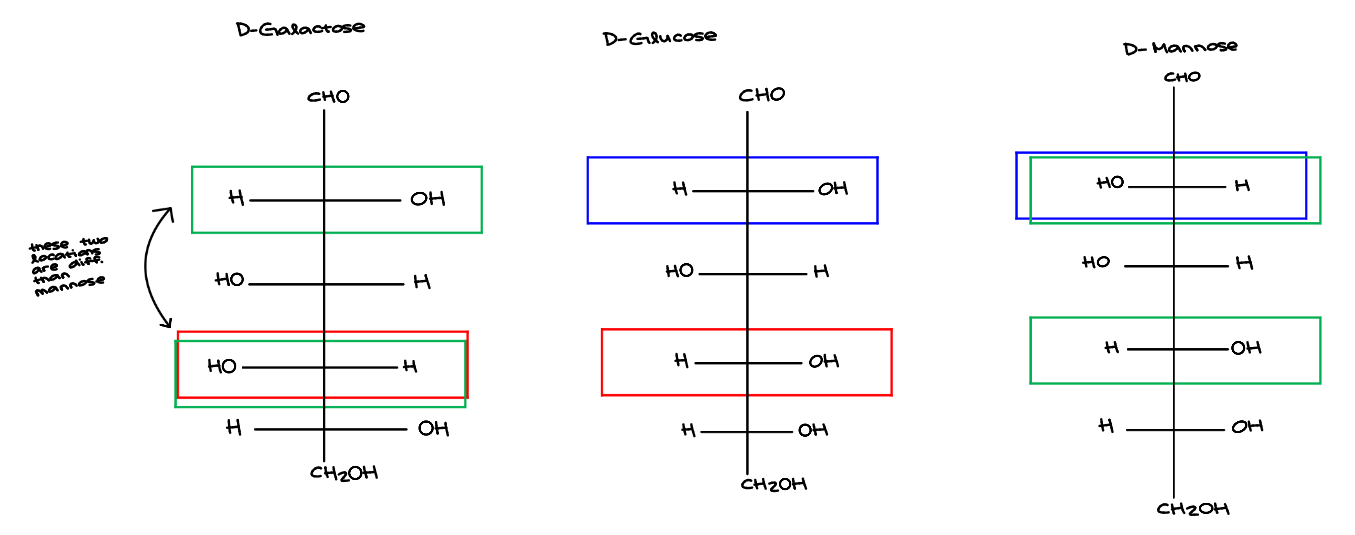
Epimers
Monosaccharides that differ by the stereochemistry about one carbon atom are called epimers
diff. (H and (-OH) localizations/arrangements
Why are Galactose and Mannose not epimers? b/c there’s more than one rearrangement
Important Aldoses
Important Ketoses
Aldose/Ketone Interconvesion
Aldose/Ketose Interconvesion is possible through the catalytic conversion of triose phosphate isomerase
Triose Phosphate Isomerase- catalyzes isomerization reaction of glyceraldehyde 3-phosphate and dihydroxyacetone phosphate
basically rearranges stuff
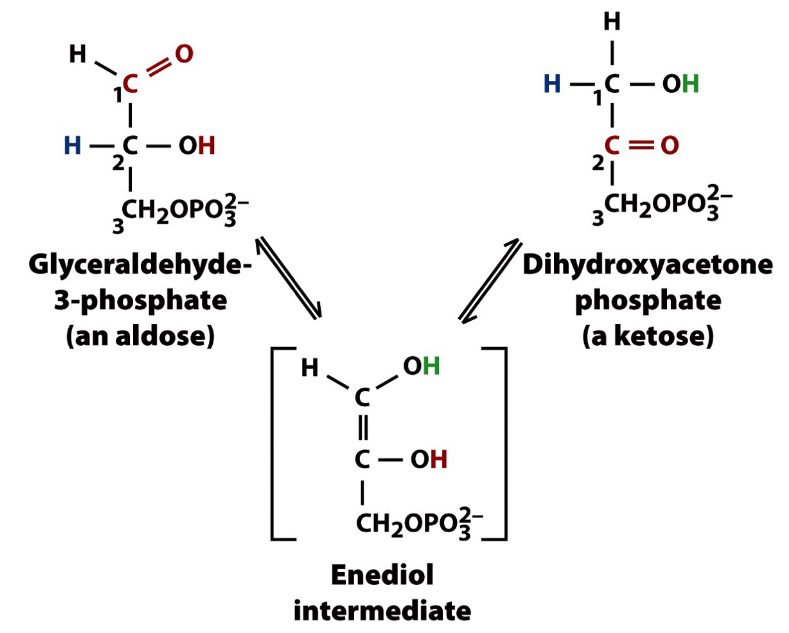
Aldose/Ketone Interconvesion Example
Glycolysis
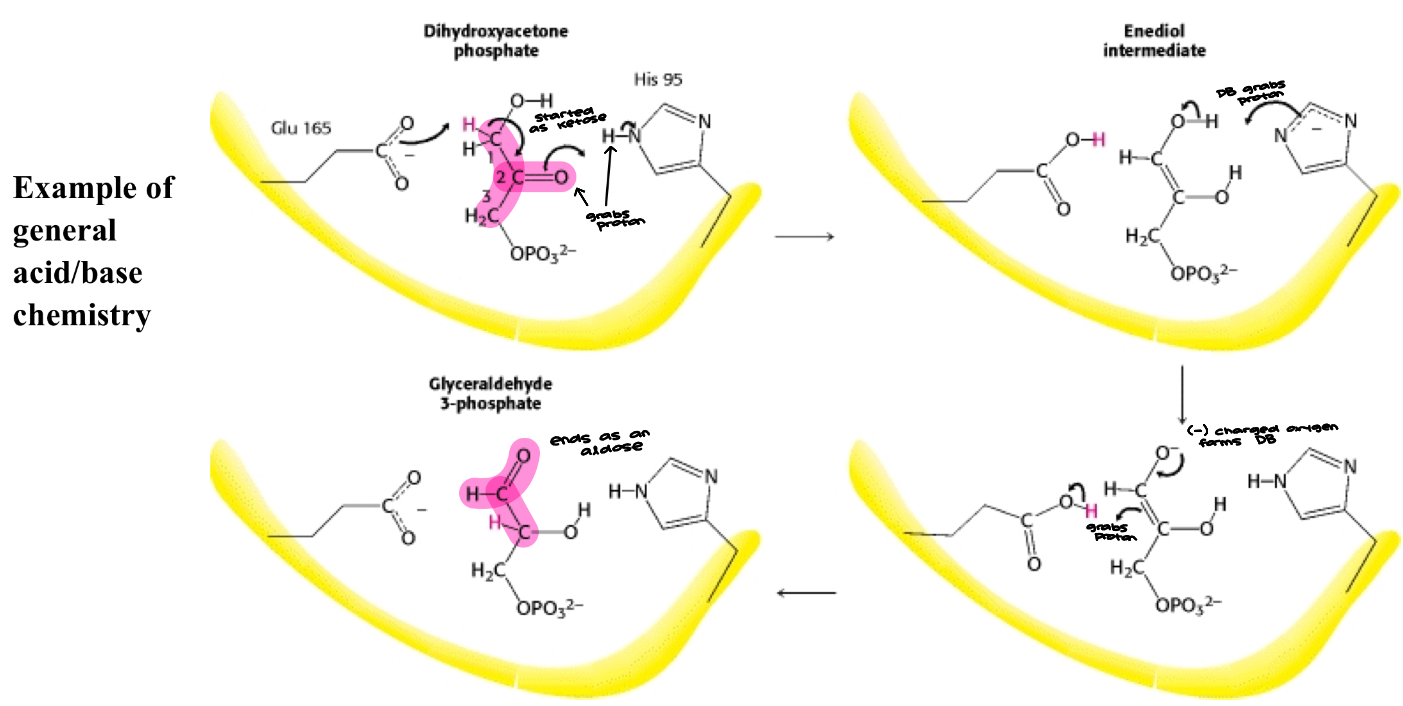
Mechanism for Triose Phosphate Isomerase
TRI is an example of a catalytically perfect enzyme
The rate of the bimolecular reaction between enzyme and substrate is diffusion controlled such that formation of product occurs as quickly as substrate and enzyme collide
Cyclization of Aldoses and Ketoses
Aldoses/Ketoses can form cyclic structures
2 important function groups play an important role in this process:
Carbonyl (aldehyde/ketone)
Alcohol (-OH) group
How does this happen mechanistically?
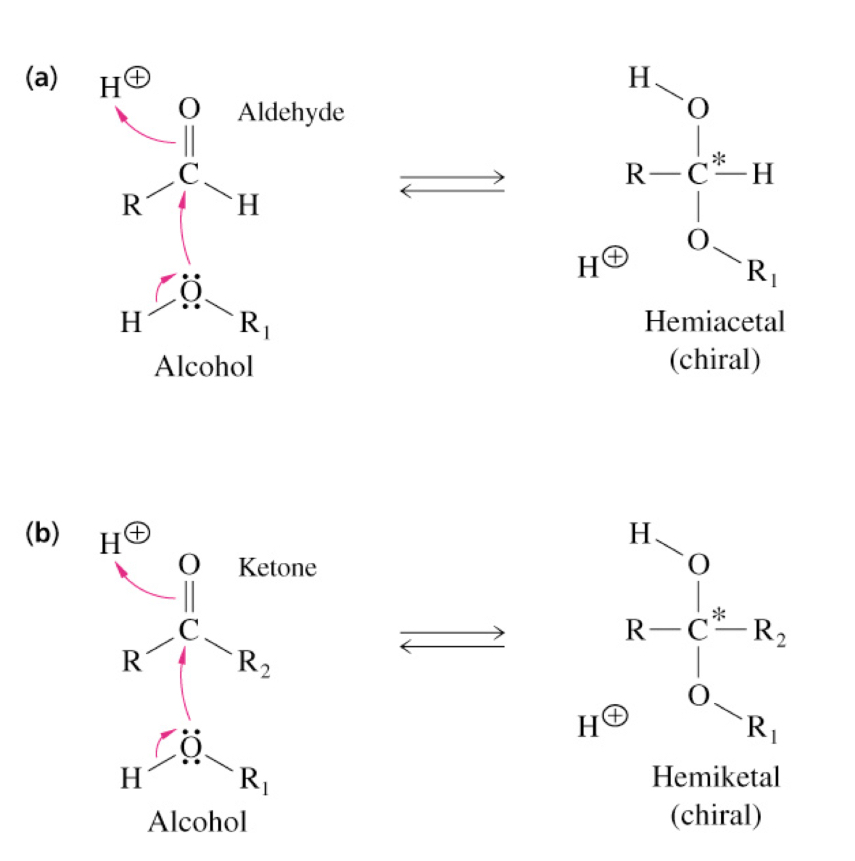
Cyclization of Aldoses and Ketoses 2
Reaction of an alcohol with:
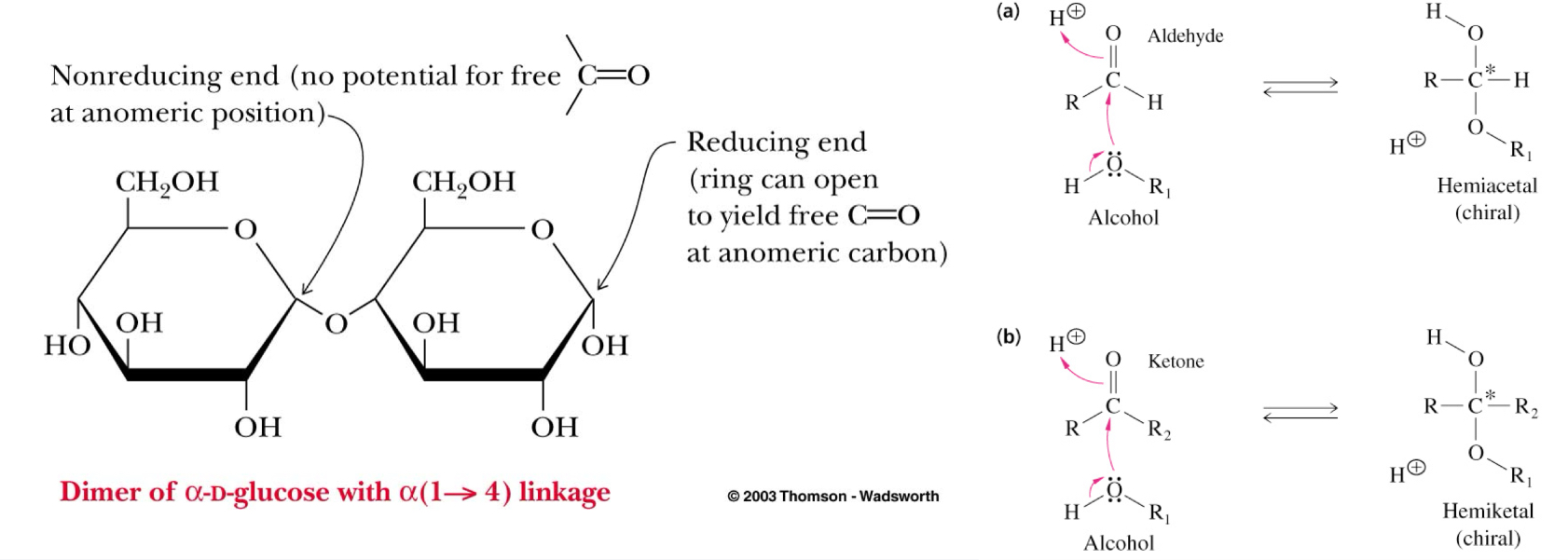
(a) An aldehyde to form a hemiacetal
(b) A ketone to form a hemiketal
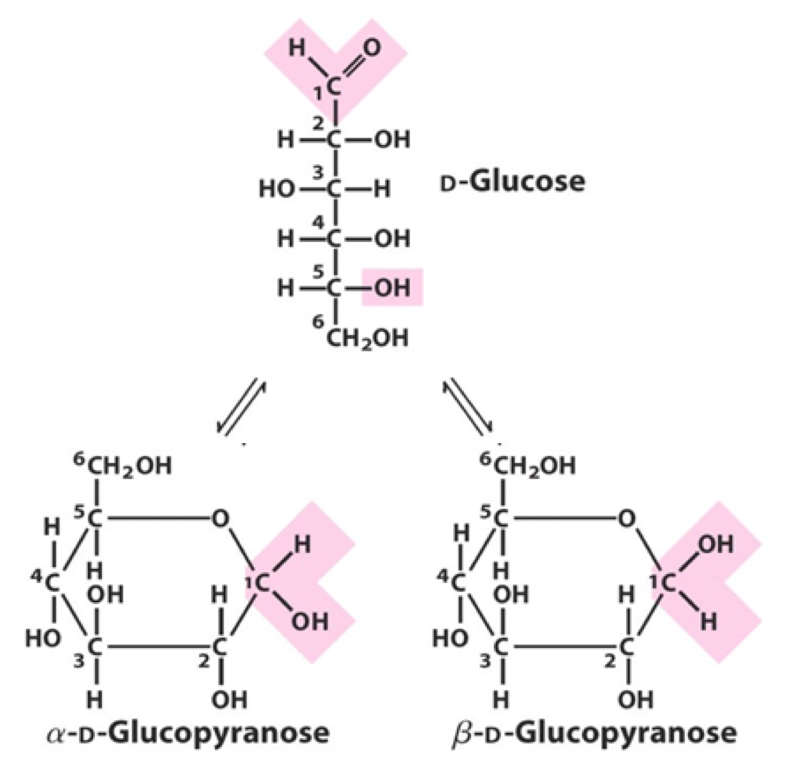
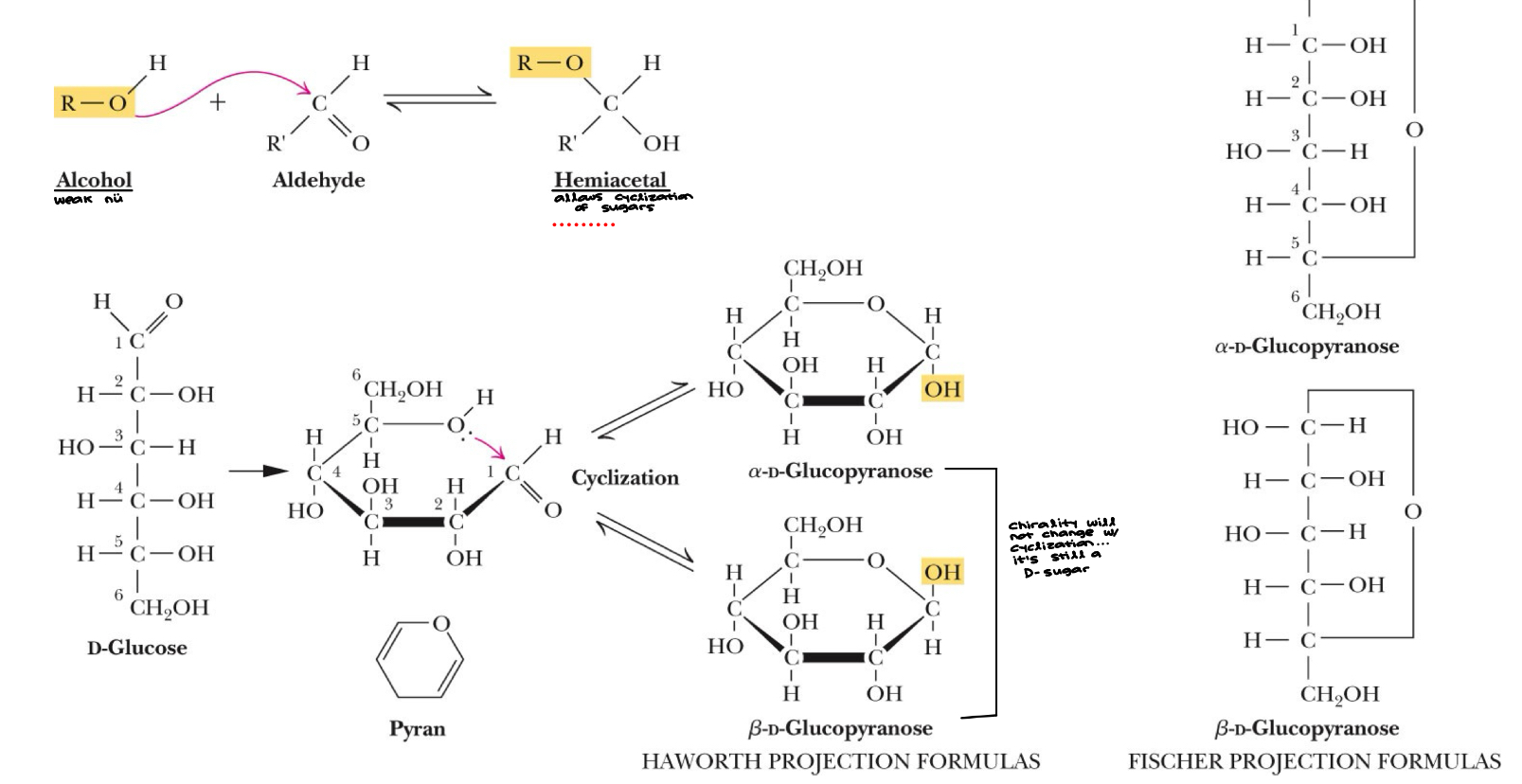
Cyclization of D-Glucose to form glycopyranose
Example: Aldose (aldehyde functional group and alcohol functional group)
Chemistry: C1 - aldehyde, C5 - alcohol - Form a 6 membered ring structure “pyranose”
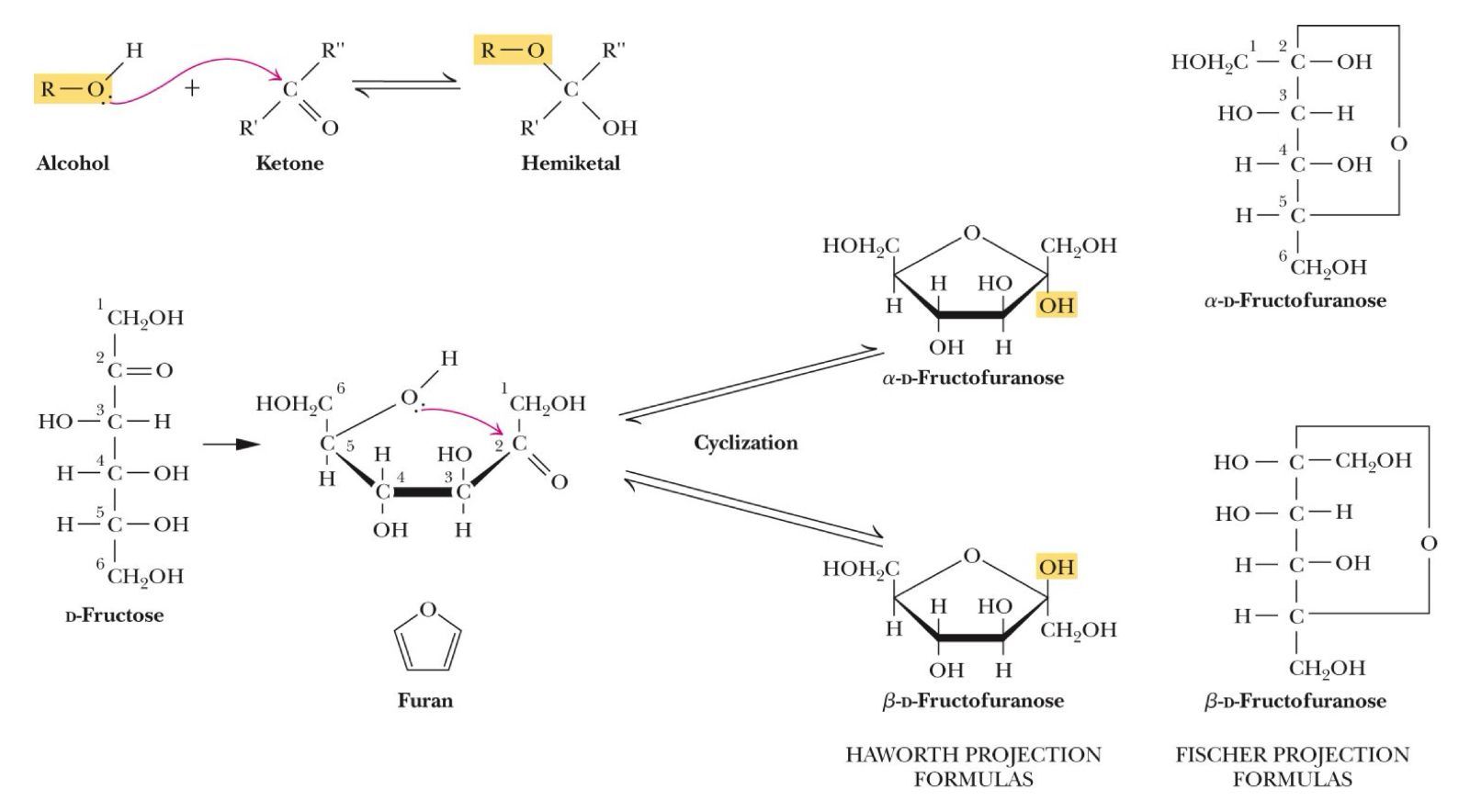
Cyclization of D-fructose to form fructofuranose
Example: Ketose (ketone functional group and alcohol functional group)
Chemistry: C2 - ketone, C5 - alcohol - Form a 5 membered ring structure “furanose”
Basic Ring Structures
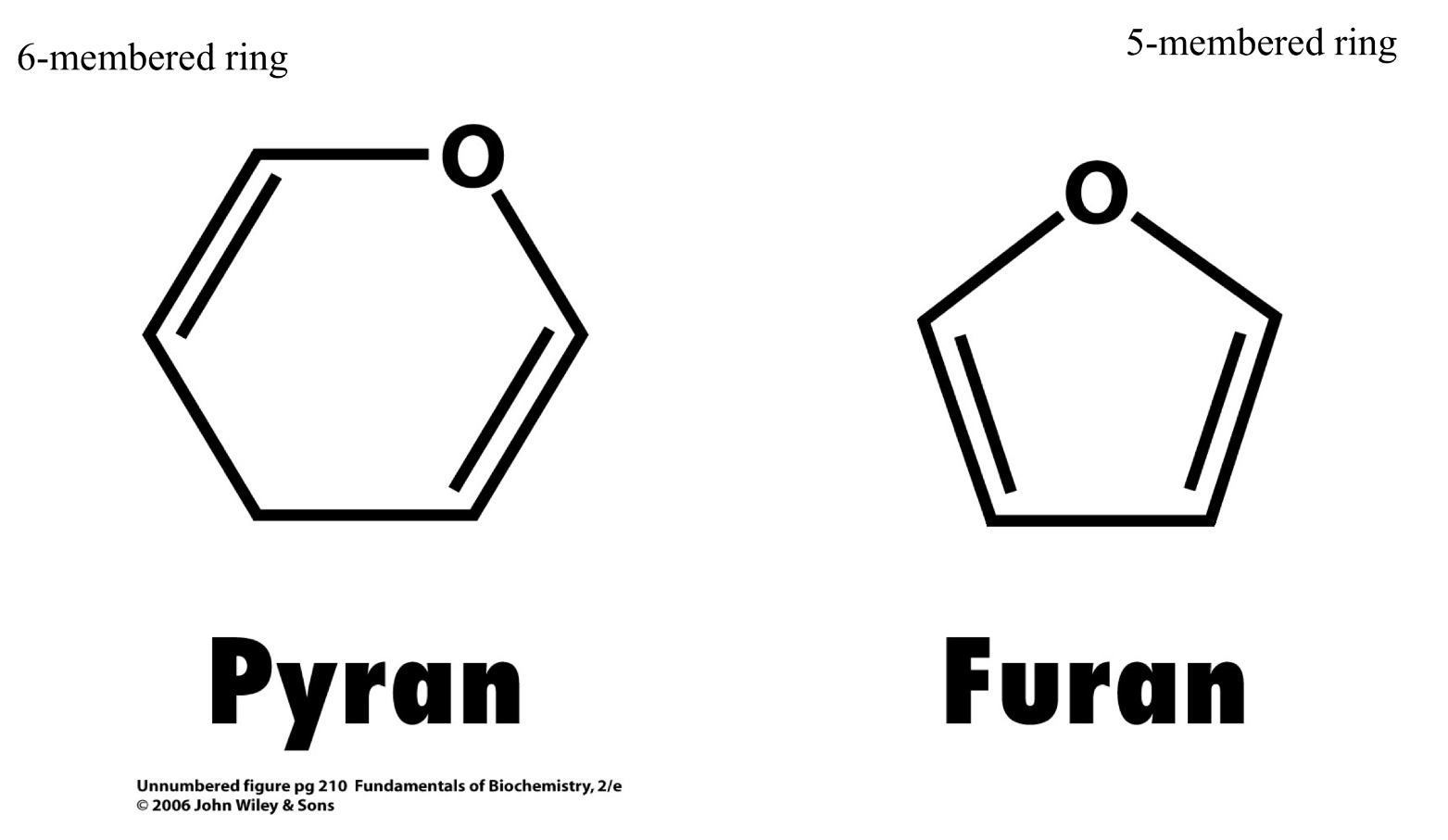
(a) Pryan and (b) furan ring systems
(a) Six-membered sugar ring is a “pyranose”
(b) Five-membered sugar ring is a “furanose”
Possibility of Multiple Cylizations
Cyclizations are not exclusive for specific forms
In many monosaccharides, there are two or more reactive hydroxyl groups that can serve to attack an aldehyde or ketone
The nature of the substituents on the carbonyl and hydroxyl groups and nature of asymmetric carbon determine pyranose vs furanose form
Aldehexose sugars prefer the pyranose structure
Ketohexose sugars prefer the furanose structure
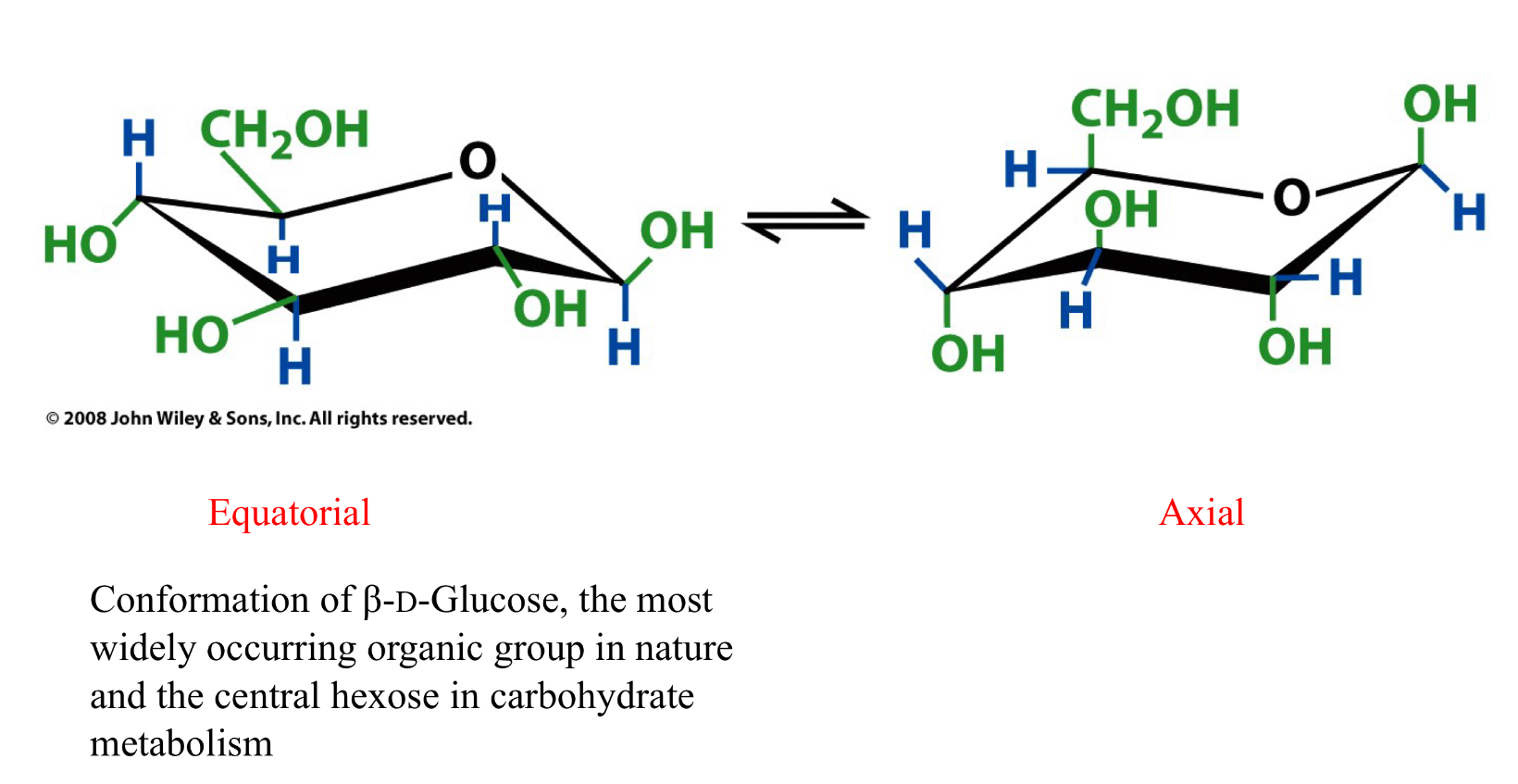
Conformations
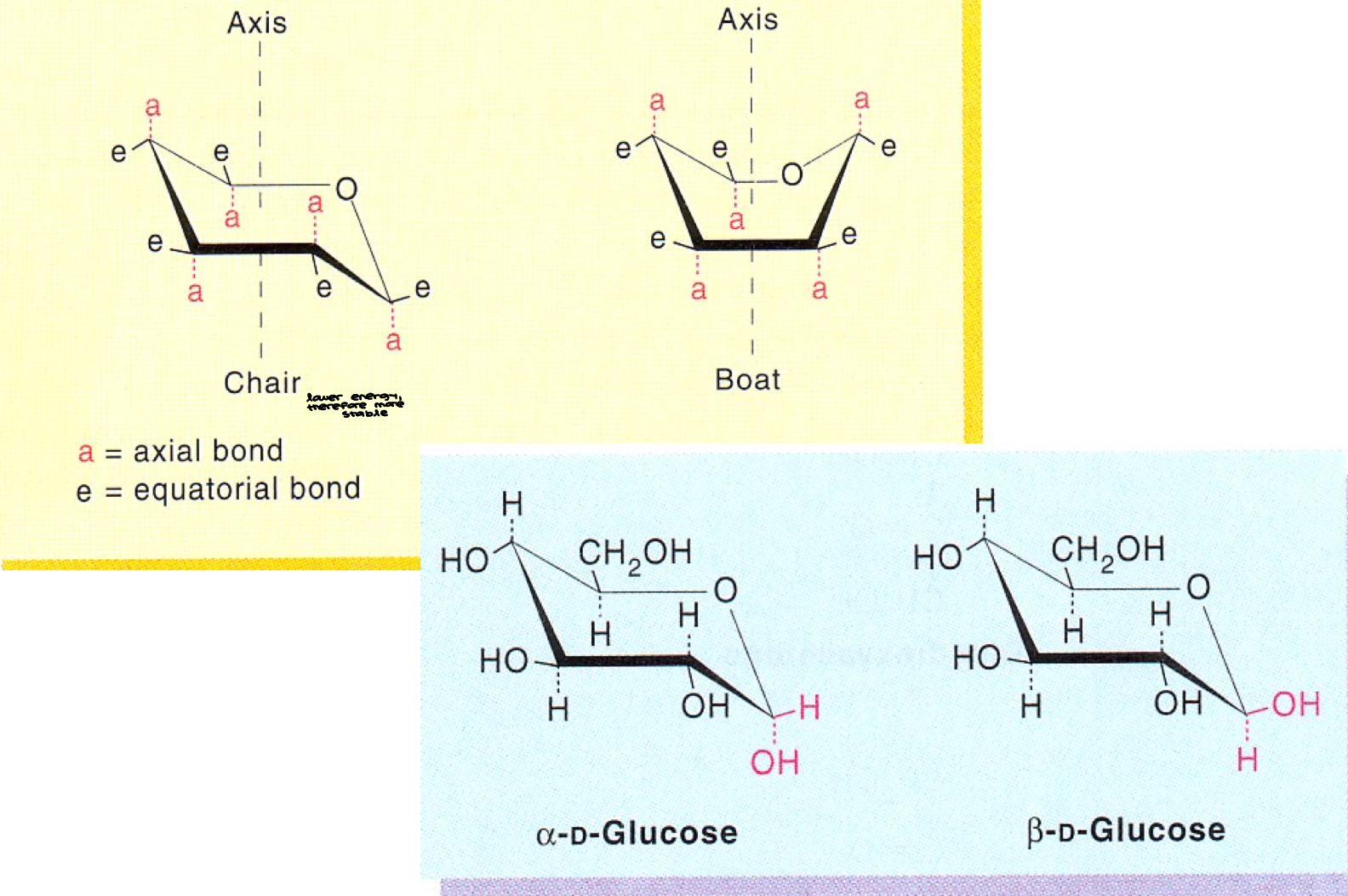
The two chair conformations of B-D-glucopyranose
Furanose and pyranose rings are NOT planar
Monosaccharides can be converted to several derivative forms
Sugars are VERY reactive
A variety of chemical and enzymatic reactions produce derivatives of the simple sugars
Some of the most common are:
- Sugar acids
- Sugar alcohols
- Deoxy sugars
- Sugar esters
- Amino sugars
- Acetals, ketals, and glycosides
Monosaccharides can be converted to several derivative forms 2
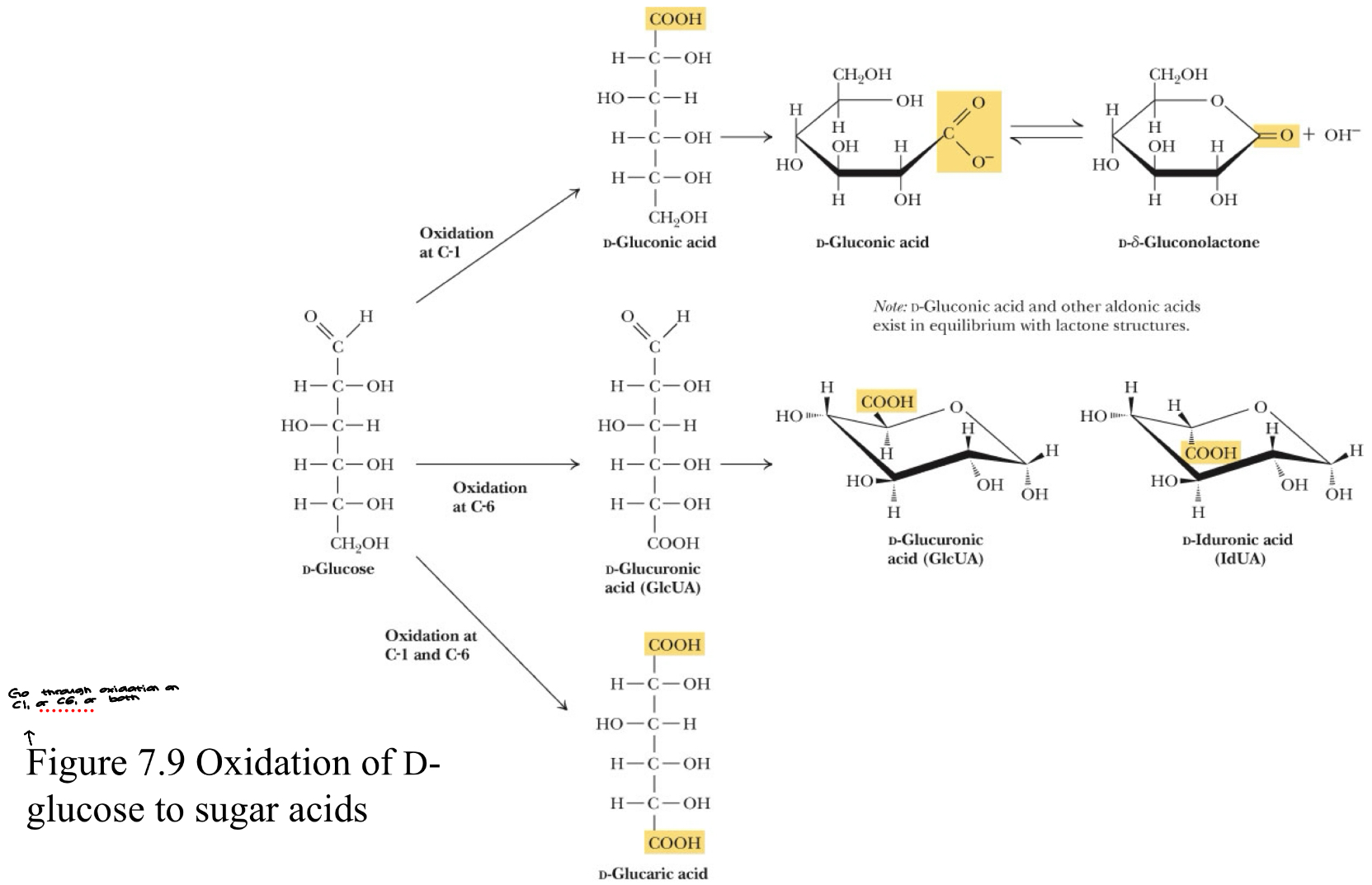
Figure 7.9 Oxidation of D-glucose to sugar acids
Sugar Derivatives
Sugars undergo reactions typical of aldehydes and ketones
These reactions have important physiological consequences
Oxidation of aldehydes produces carboxylic acids
Oxidation of primary alcohols yield uronic acids
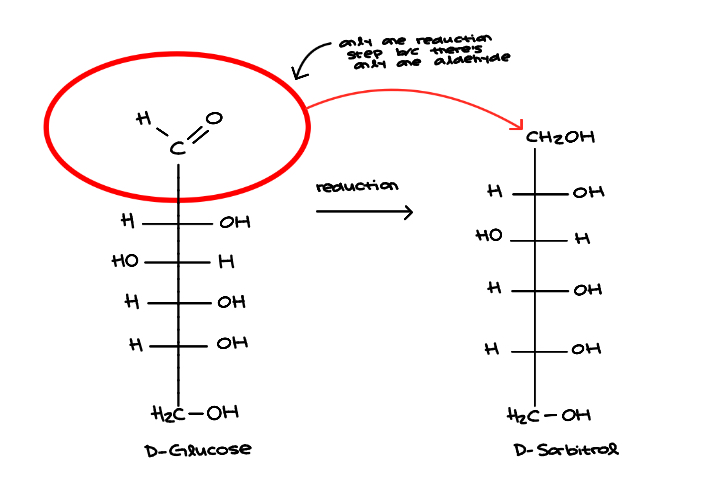
Reduction
Aldoses and ketoses produce ribitols
Hydroxyl groups replaced by hydrogens
Amination- replacing of -OH groups with -NH2
Sugar Derivatives- Oxidation
Oxidation
Reducing Sugars- sugars containing a free aldehyde
Oxidation of
the aldehyde group
aldonic acid (“onic acid”)
Oxidation of
primary alcohol group
uronic acid (“uronic acid”)
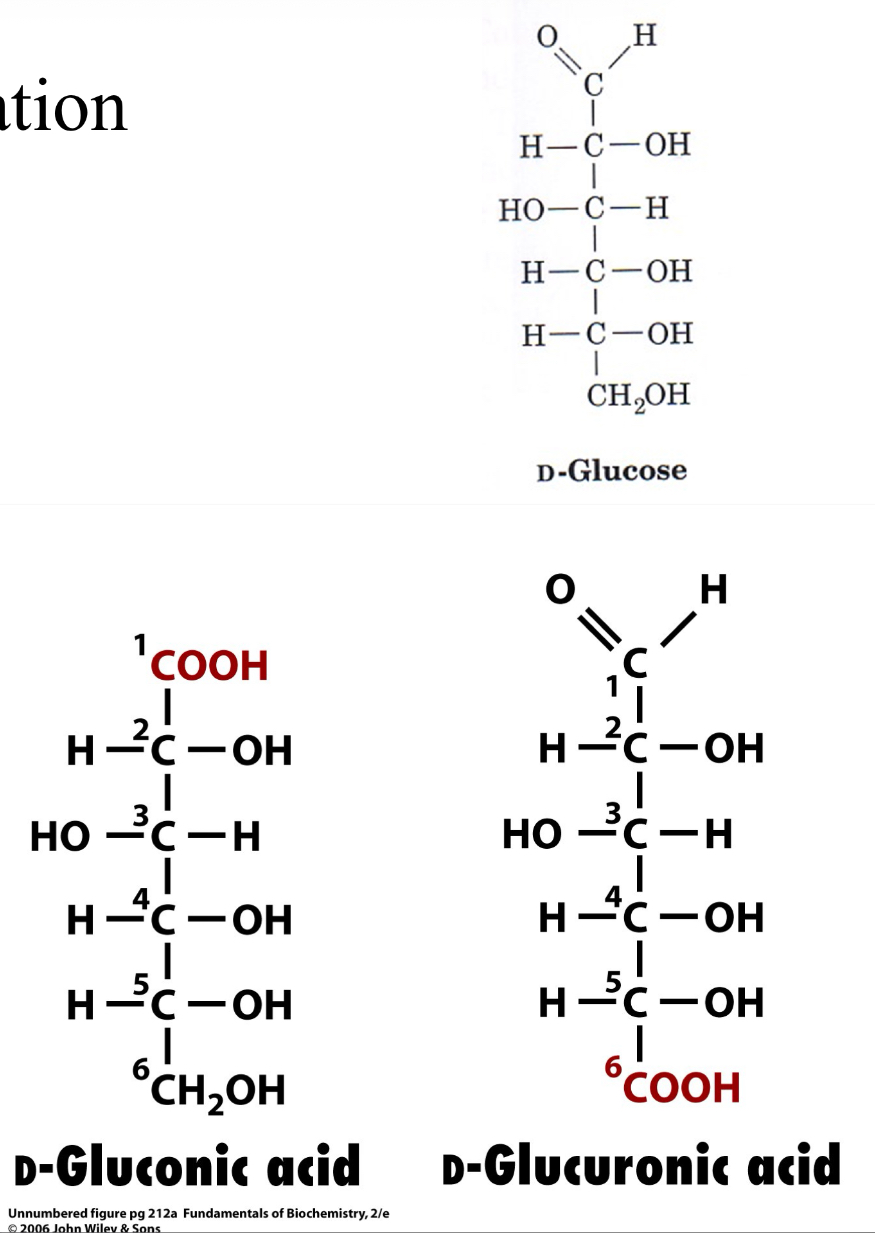
Sugar Derivatives- Reduction (SLIDE 28)
Reduction (gain electrons)
Aldehydes are reduced to form alcohols
Sugar Alcohols:
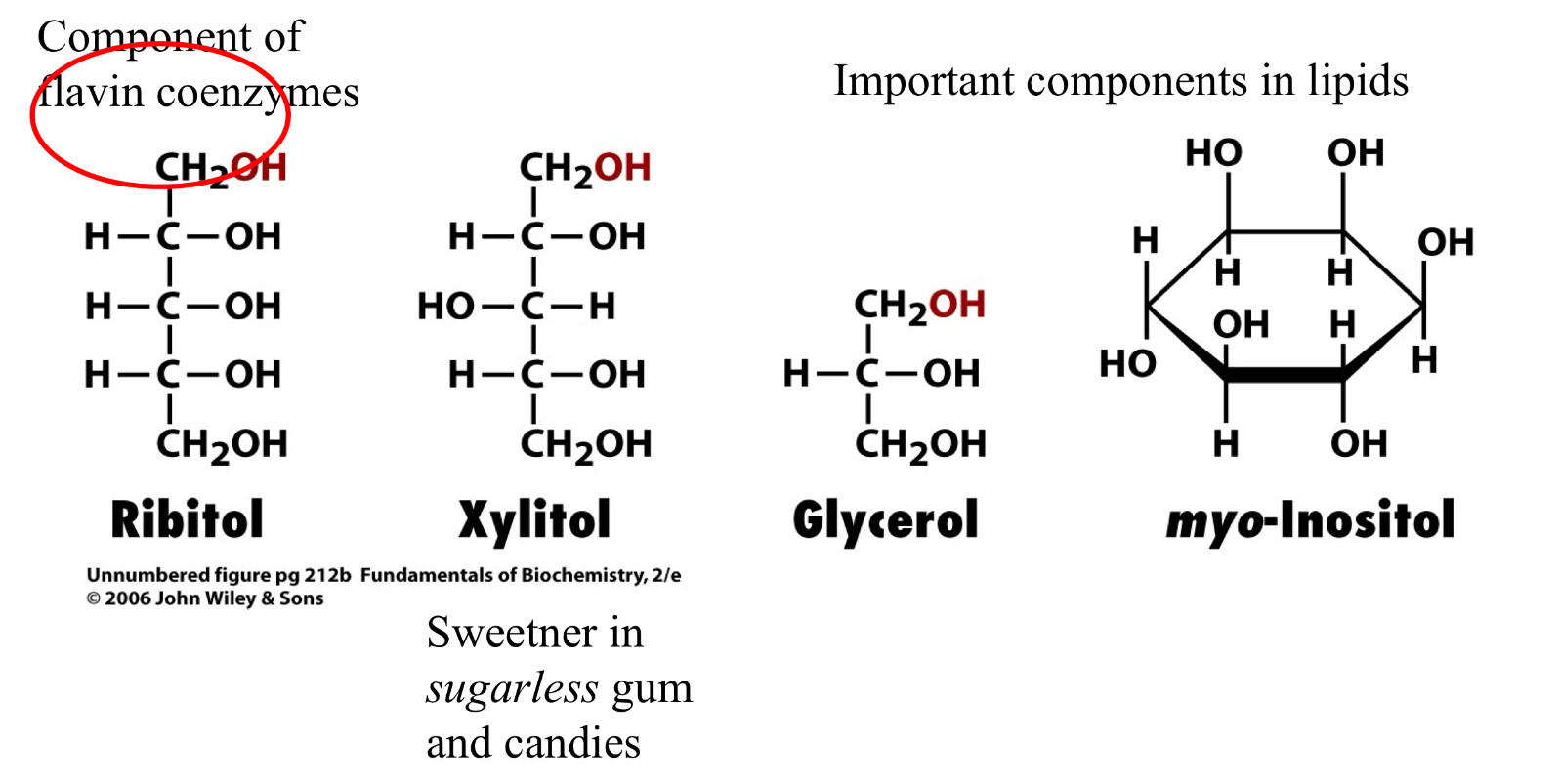
Sugar Alcohols: Xylitol
Sweeter in sugarless gum and candies
Sugar Alcohols: Glycerol
Important components in lipids
Sugar Alcohols: myo-Inositol
Important components in lipids
Amination
Important constituent of glycoproteins and glycolipids
Proteins and lipids with covalently attached carbohydrates
Many possible functions:
Enzymes
Transport proteins
Receptors
Hormones
Structural proteins
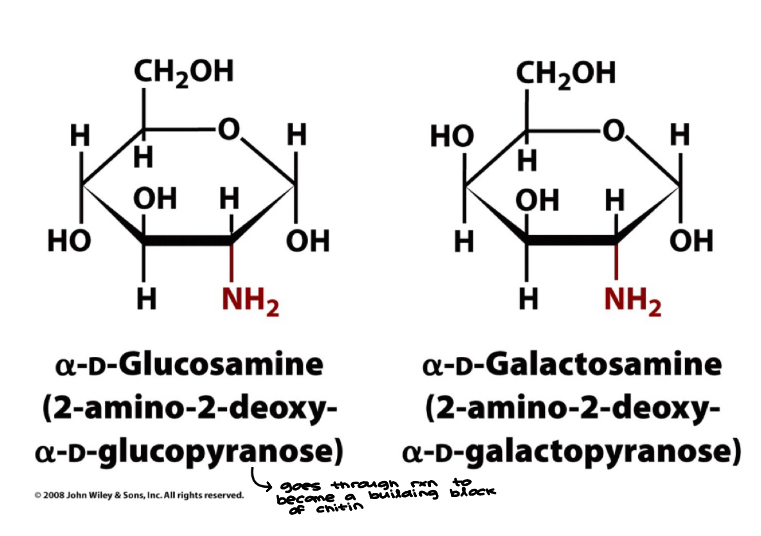
Cyclic Carbohydrates
A hydroxyl end can react with the carbonyl of the aldehyde or ketone within a monosaccharide to form cyclic compounds
All monosaccharides with five or more carbon atoms in the backbone occur predominantly in the cyclic structure
a: C1-OH down, B: C1-OH up
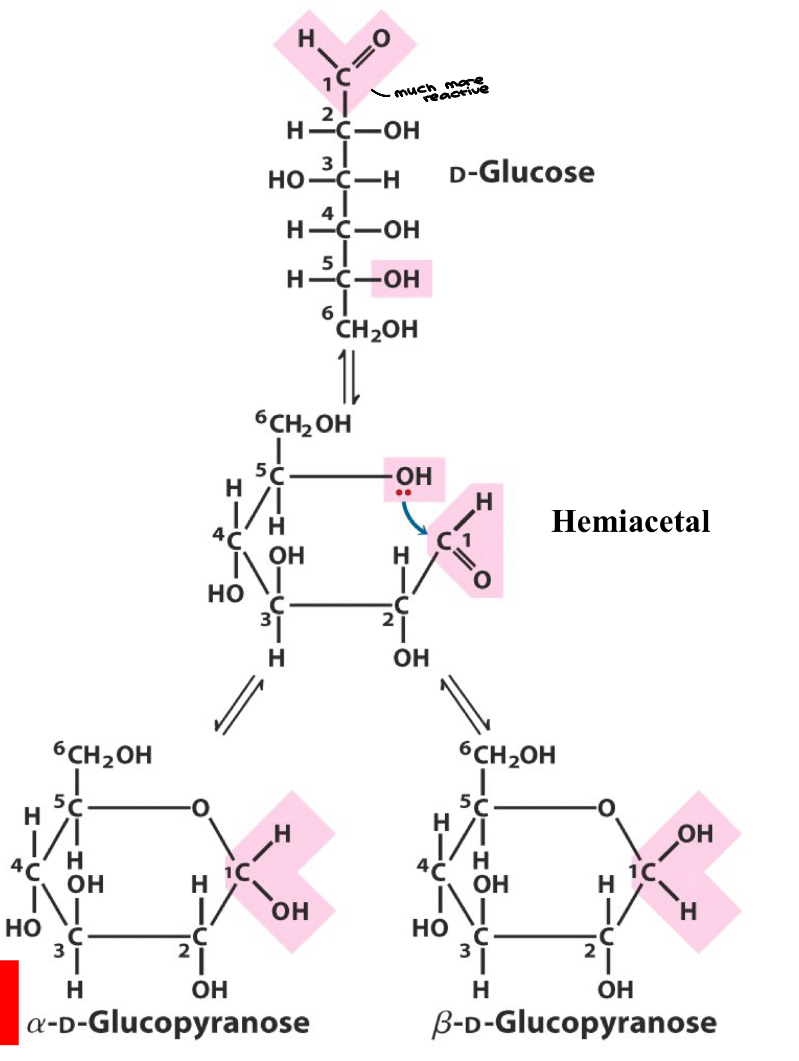
Anomeric Carbon
Monosaccharides exist almost entirely as five- and six-membered rings
anomeric carbon: the carbonyl carbon used for cyclic formation
anomers: carbohydrates that differ in configuration only at their anomeric carbons
a: C1-OH down, B: C1-OH u[
Important nature of anomeric carbon as it relates to glycosidic bond formation joining two monosaccharides forming disaccharides and polysaccharides
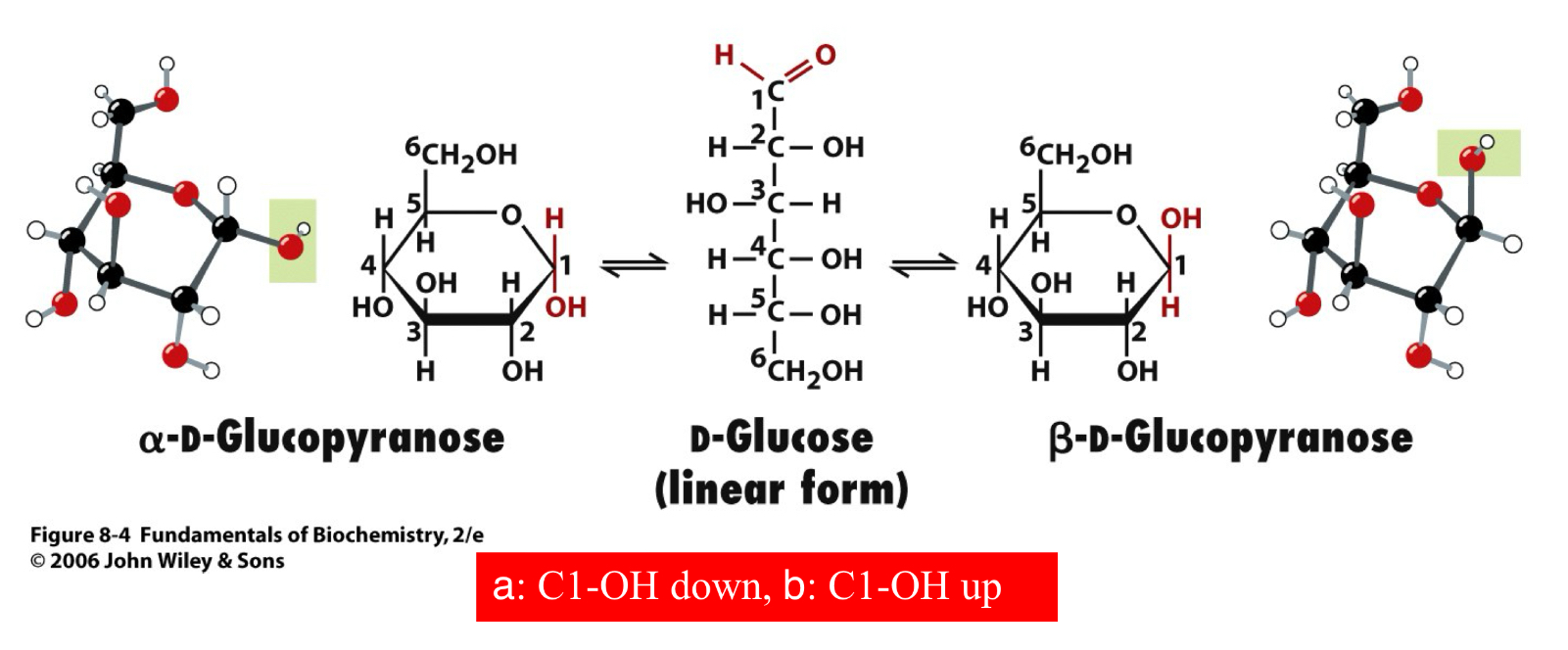
Glycosidic Bond
Glycoside: a carbohydrate in which the -OH of the anomeric carbon is replaced by -OR
those derived from furanoses are furanosides; those derived from pyranoses are pyranosides
glycosidic bond: the bond from the anomeric carbon to the -OR group, can be a or B-glycosidic bonds
Glycosidic Bond 2
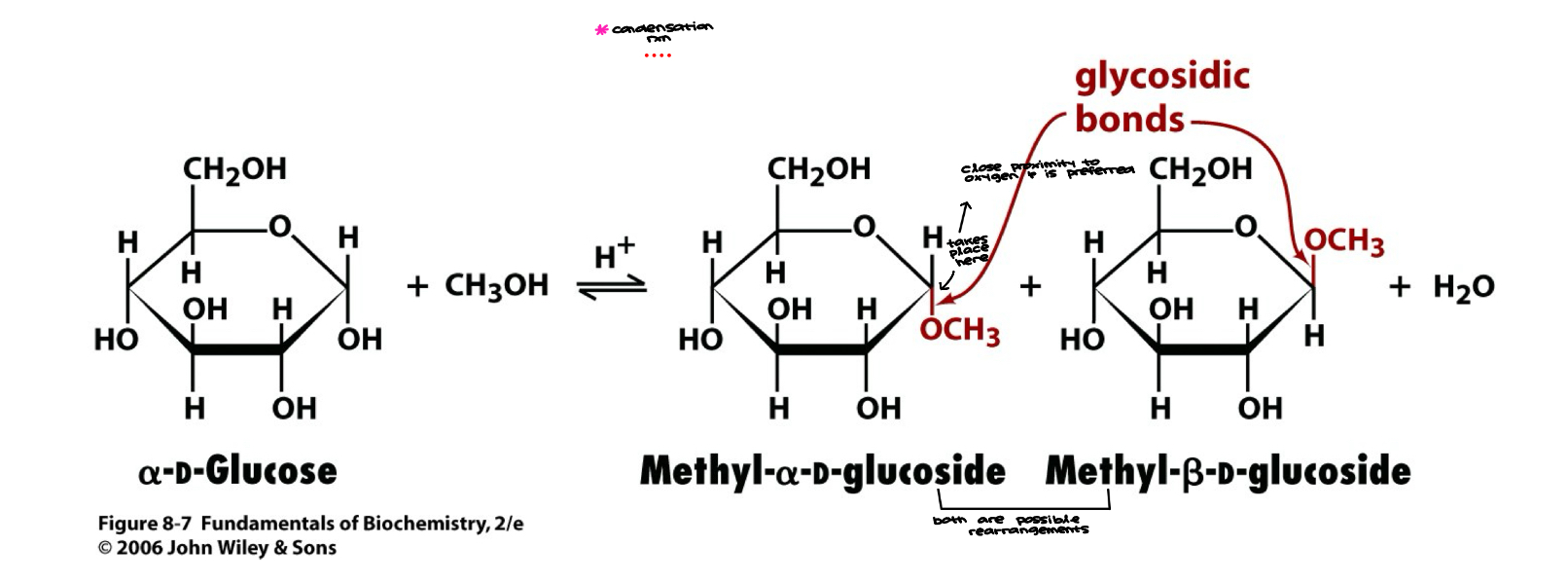
Glycosidic Bonds
Important bond formation in linking monosaccharides to form polysaccharides
Have properties of hydroxyls (OHs/alcohols)
If there’s a free anomeric carbo, then it’s capable of reducing reactions
Polysaccharides
Also called glycans
Consist of monosaccharides linked together by glycosidic bonds
formation of glycosides: The anomeric carbon of a sugar combines (condenses) with an alcohol
a or B-glycosides
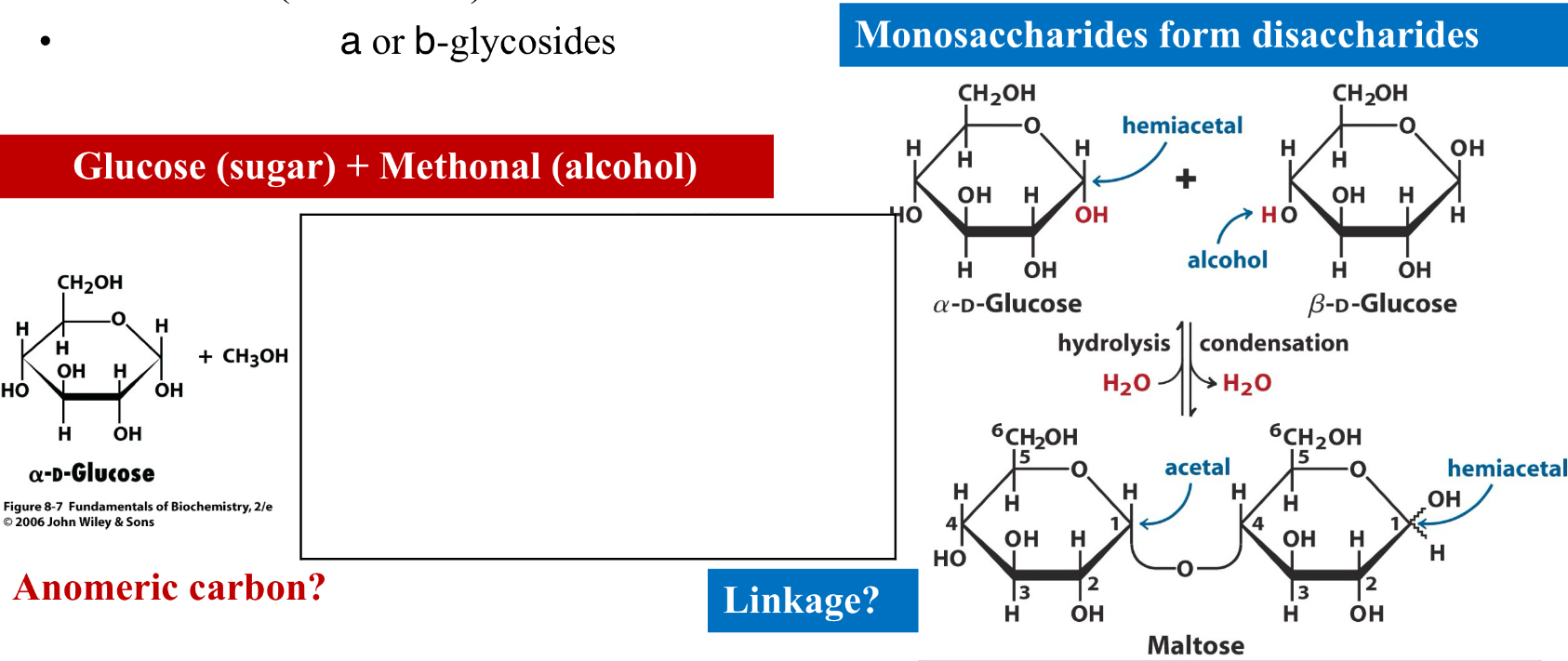
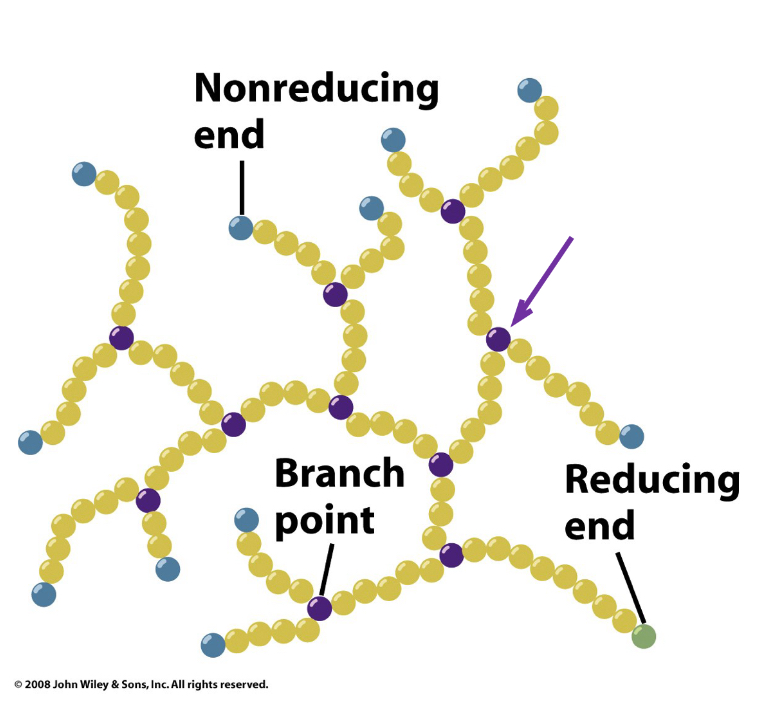
Reducing Ends
Monosaccharides have reducing ends
Upon formation of a glycosidic bond, the anomeric carbon becomes non-reducing
A reducing end can still participate in ring opening, yielding a free carbonyl
Important Di- & Polysaccharides
Disaccharides
Lactose
Sucrose
Polysaccharides
Cellulose
Chitin
a-amylose
Lactose
Disaccharide
Occurs in milk
Composed of galactose and glucose
Both galactose and glucose exist as a B-anomer
Linkage is B (1 → 4)
Glucose molecule contains a reducing end, therefore lactose is a reducing sugar
Quick energy source and can break/release two sugars
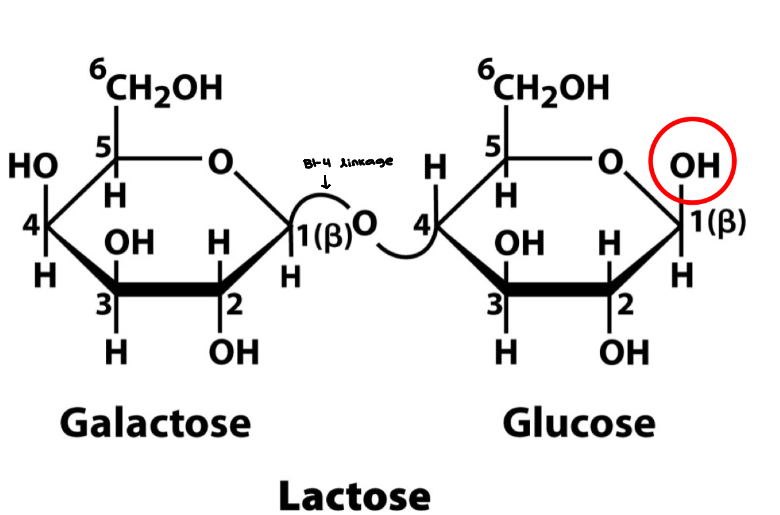
Lactose 2
Lactose intolerance?
Symptoms: gas, belly pain, bloating
What causes it?
Deficiency in enzyme lactase, not enough is produced in the small intestines to aid in the breakdown of glucose
Not a food allergy
What is lactose?
Glycoside hydrolase that is involved in the hydrolysis of the disaccharide lactose into constituent galactose and glucose monomers
Asian lineages that don’t produce or drink milk tend to lack lactose, making them lactose-intolerant
Another example with Russians for ethanol and not being able to stomach alcohol
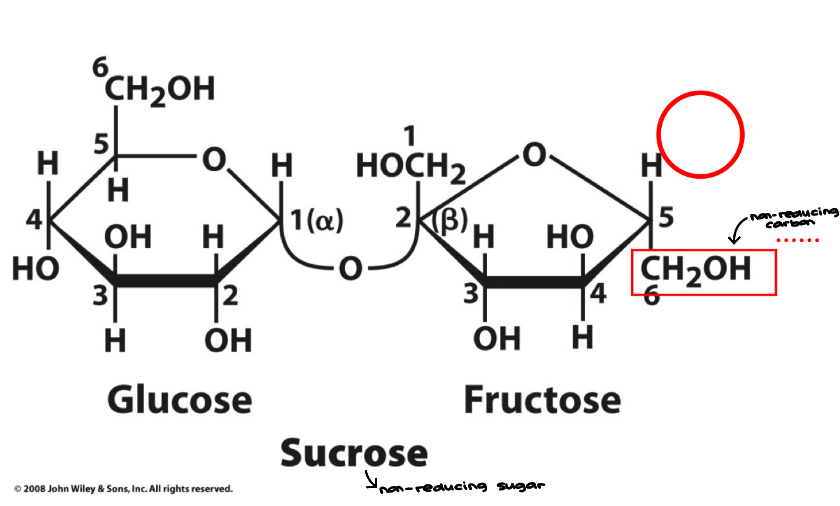
Sucrose
Disaccharide
Most abundant disaccharide
Table sugar
Composed of glucose and fructose
Glucose is in the a-anomeric form and fructose is in the B-anomeric form
Linkage is a (1 → 2)
Fructose has a non-reducing end, therefore sucrose is a non-reducing sugar '
Sugar canes are an example… highly prevalent in Florida
7.4 What is the structure and Chemistry of Polysaccharides
Functions: storage, structure, recognition
Nomenclature for polysaccharides is based on their composition and structure
Homopolysaccharide- a polysaccharide that contains only one kind of monosaccharide
same units of monomeric sugar copied # number of times in a polymer
Heteropolysaccharide- a polysaccharide made of several monosaccharides
diff. sugars coexisting in a polysaccharide
Starch and glycogen are storage molecules
Chitin and cellulose are stuctural molecules
Cell surface polysaccharides are recognition molecules
Cellulose (know structure)
Polysaccharide- homopolysaccharide
Primary structural component in plants
Accounts for half the carbon in the biosphere
Linear molecule of up to 15,000 D-glucose residues
Glucose molecules are in the B-anomeric form
Linkage is B (1 → 4)
Building block of plant cell walls
Most commonly observed and most widely distributed in nature
Polymer of glucose
Extremely steep polymer, it is building into self-assembling fibers… fibers is what provides stability to plants & allows them to grow, withstand harsh environmental conditions and high temp.
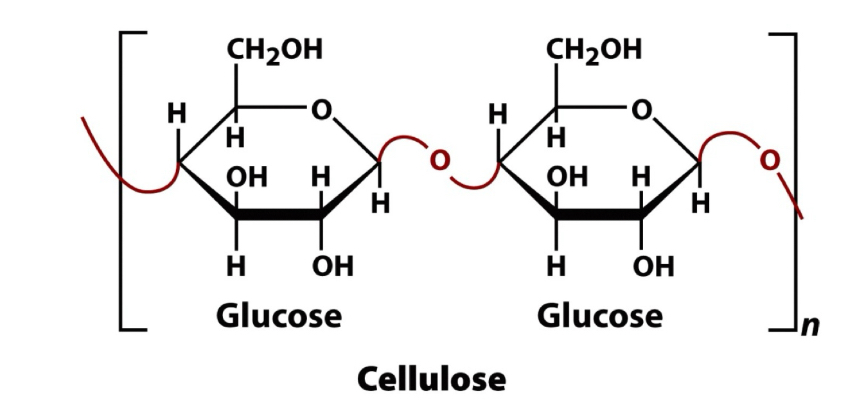
Cellulose 2
Parallel cellulose chains form sheets with interchain H-bonds
Stacks of the fibers occur having exceptional strength and are water insoluble
Cellulases are enzymes within symbiotic microorganisms found in herbivores that hydrolyze the B (1 → 4) linkages of cellulose
Humans can not digest cellulose, fiber
Animals that consume plants have cellulases, enzymes in gums that can break down cellulose. We don’t have these enzymes
Cellulose is still a very important component of our diet. Helps us have proper digestive processes in our stomach. Important to consume green food… if you don’t have access/don’t have enough, there are fiber supplements
Fibers
Classification of dietary fibers:
Based on their ability to dissolve in water
Soluble
Insoluble
Act by changing the nature of the contents of the GI tract and by changing how other nutrients and chemicals are absorbed
Uses for fiber:
nutritional purposes
treatment of various gastrointestinal disorders
health benefits as lowering cholesterol levels, reducing risk of colon cancer, and losing weight
Fiber supplements
Benefiber
Metamucil
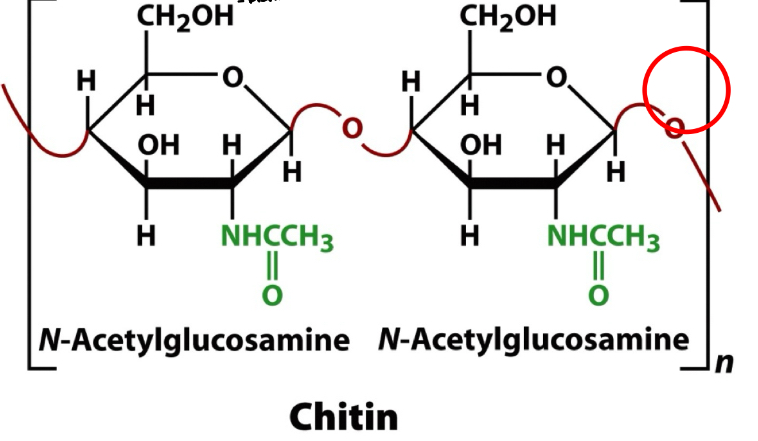
Chitin (know structure)
Polysaccharide- Homopolysaccharide
Principal structural component of the exoskeletons of insects, crustaceans, & spiders, & in the cell walls of most fungi and algae
Homopolymer of N-acetylglucosamine
N-acetylglucosamine molecules are in the B-anomeric form
Linkage B (1 → 4)
very interesting monomeric structure b/c in its monomer sugar residues you have amide
if cellulose is transparent, chitin will have dark appearance
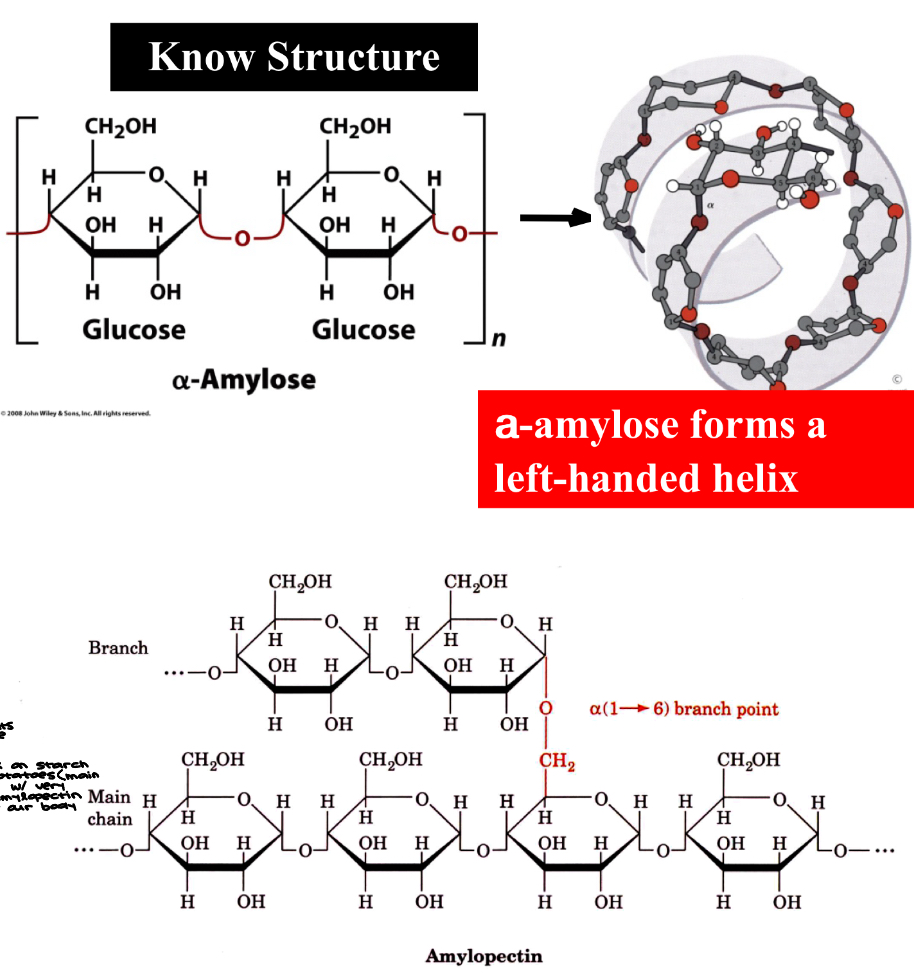
Starch (know structure)
Starch- storage polysaccharide in plants with mixture of a-amylose and amylopectin
starch is a combination of these 2 polymers
in both cases, we have glucose linked with a a-1,4 glycosidic bonds
a-amylose is a linear polymer of a (1 → 4) linkages
Isomer of cellulose
Amylopectin can contain 10^6 glucose molecules
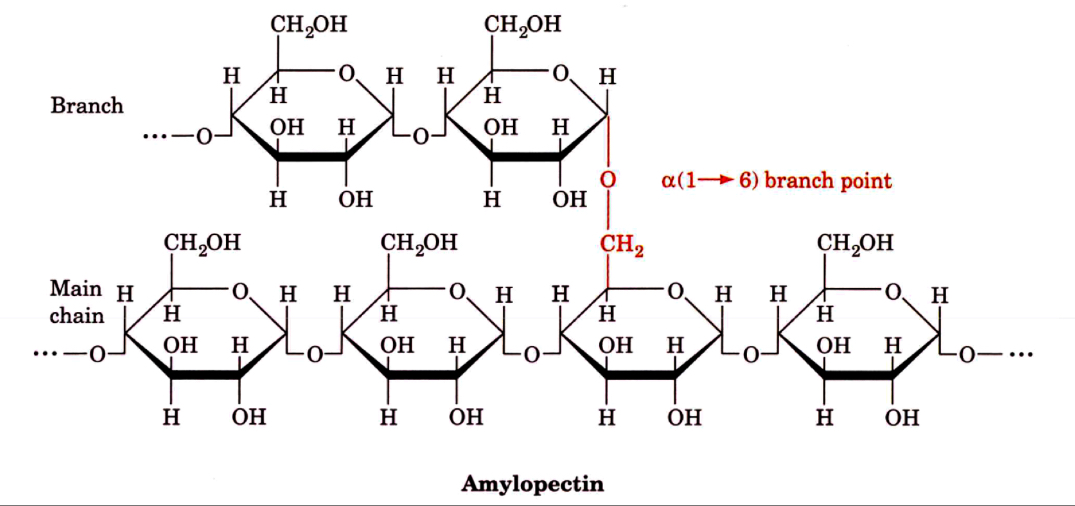
Consists mainly of a (1 → 4) linkages, but can form a branched molecule with a (1 → 6) linkages
people that work on starch prefer to grow potatoes (main source of starch) with very small amounts of amylopectin b/c it’s harder for our body to digest
Glycogen
Storage polysaccharide of animals
Present in all cells
Mainly skeletal muscle and the liver
liver breaks down glycogen and produces monomeric sugars when we need energy
More highly branched than amylopectin
important for high stability of glycogen in liver
needs higher energy to digest polymer and produce monomeric sugars
Amylopectin & Glycogen
a (1 → 6) branch points
amylopectin: every 24 to 30 glucose residues
glycogen: every 8-12 glucose residues
Starch
Starch is processed to produce many of the sugars in processed foods
Glycogen and amylopectin have the same structure, but the former has about one branch point per ten 1,4-alpha bonds, compared to about one branch point per thirty 1,4-alpha bonds in amylopectin
Amylopectin is synthesized from ADP-glucose while mammals and fungi synthesize glycogen from UDP-glucose
Starch is the most common carbohydrate in the human diet
Starch is a great form of energy, but when eaten in too high of quantities, results in weight gain
Some starch turns to sugar very quickly raising blood glucose faster than table sugar
Breakdown of Starch
taste sweet? monomeric sugars are hydrolyzed
Amylase (starch hydrolyzed by this enzyme that is present in saliva)
Enzyme is saliva that hydrolyzes the a (1 → 4) glycosidic bonds of starch
a-glucosidase
Hydrolyzes one glucose residue at a time
Debranching enzyme
Hydrolyzes a (1 → 4) and a (1 → 6) glycosidic bonds
Glycogen Breakdown
Glycogen phosphorylase- cleaves glycogen’s a (1 →4_ bonds sequentially inward from non-reducing ends
Glycogen debranching enzyme- cleaves a (1 → 6) bonds
Why carb-free diets can be dangerous:
Brain functions on glucose! Need certain levels of glucose to maintain healthy brain function
Other issues results from low carbs potentially:
Kidney stones- produce more uric acid creating solubility issues with urine expulsion
Gout- high levels of uric acid are also associated with crystallization and association with joins, causing painful inflammation
So the dietary answer:
For the general population, a balanced diet with appropriate exercise is the best approach at getting healthy
Carbohydrate calories should be 20% of total energy consumption
Keto diet (unsat. fats… no sugar, more fats (butter))
largely excludes carbs
dangerous diet b/c body will entirely change metabolism… sugar is important
Kurouski lab: mice on vegan diet, keto diet, American diet
Interested in relationship b/w neurodegeneration and diet, not stress
inject 6-hydrozopamine in stratum and make them rotate… number of rotations will indicate degree of degeneration of neurons because the more neurons that are degenerated in one area, the greater the mice will rotate
mice that were on keto diet developed Parkinson’s much faster
saturated fats, not cholesterol (trigger neurodegeneration)
activated MI is what kills cells in the brain
mice on keto diet have extremely large liver because it needs enzymes to metabolize high amounts of fats consumed… food looks like toothpaste
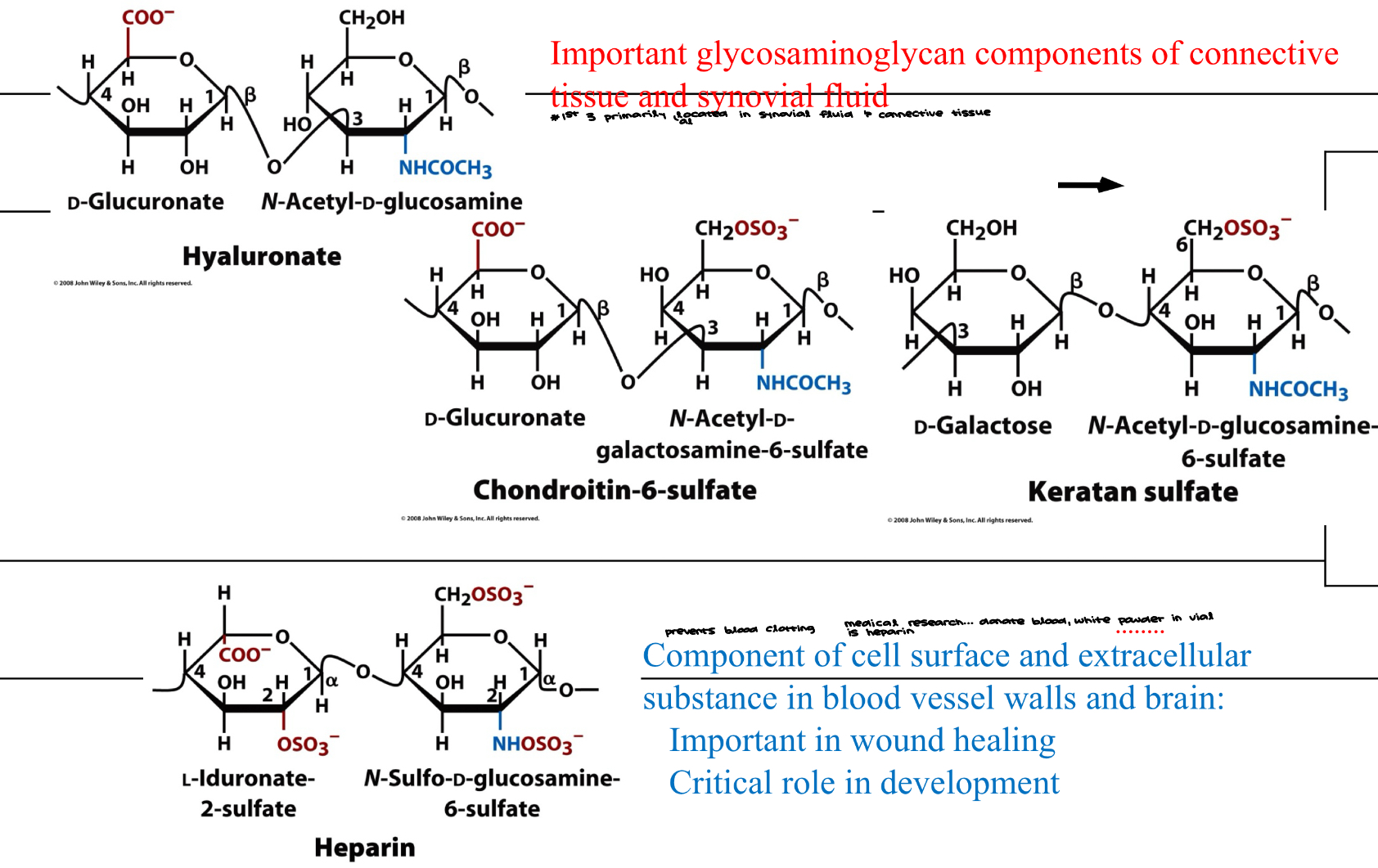
Glycosaminoglycans
Important glycosaminoglycan components of connective tissue and synovial fluid
Component of cell surface and extracellular substance in blood vessel walls and brain: Important in would healing, critical role in development
heterosugars have more complex structures
sulfanated glycosaminoglycans are very important… present in seaweed and their consumption can be useful to treat lots of diseases related to retina
Glycoproteins
Glycosylated proteins provide exclusive properties to proteins, primarily immunoproception
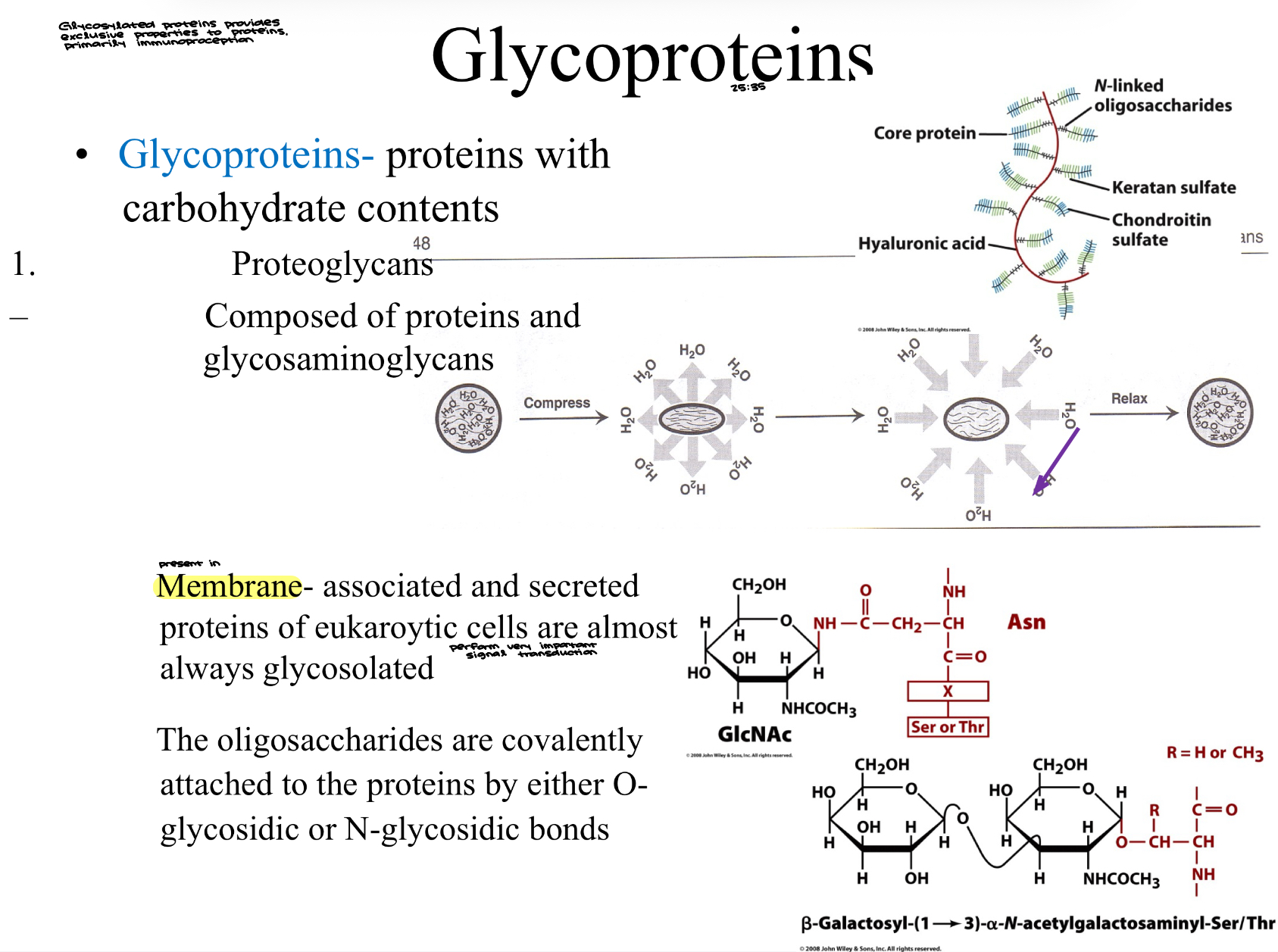
Glycoproteins- proteins with carbohydrate contents
Proteoglycans
Composed of proteins and glycosaminoglycans
Membrane- associated and secreted proteins of eukaryotic cells are almost always glycosolated
perform very important signal transduction
The oligosaccharides are covalently attached to the proteins by either O-glycosidic or N-glycosidic bonds
Glycoproteins 2
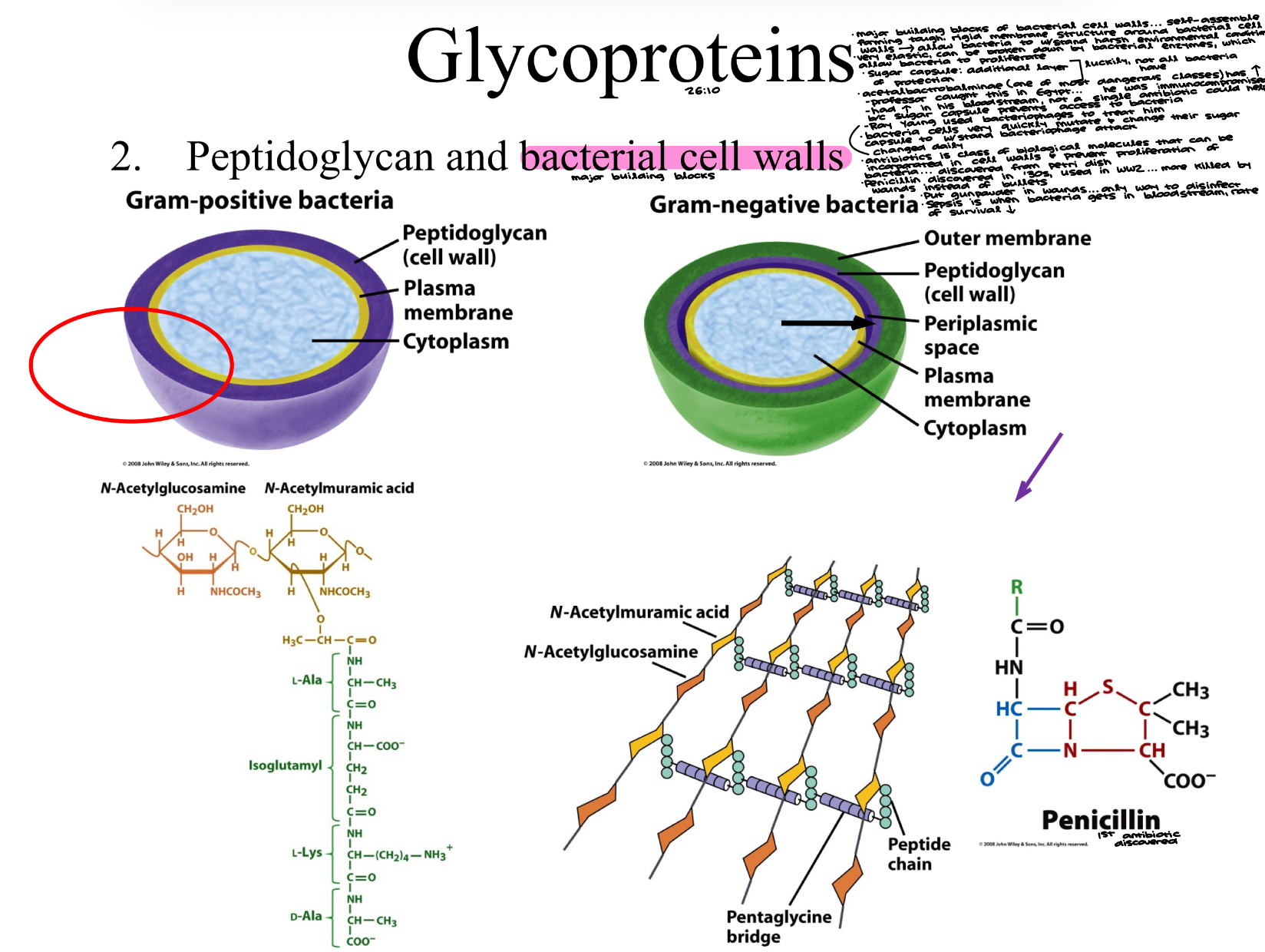
Peptidoglycan and bacterial cells walls
major building blocks of bacterial cell walls… self-assemble forming tough, rigid membrane structure around bacterial cell walls → allow bacteria to withstand harsh environmental conditions
very elastic, can be broken down by bacterial enzymes, which allow bacteria to proliferate
sugar capsule: additional layer of protection (luckily, not all bacteria have)
acetalbactrobalminae (one of most dangerous classes) has sugar capsule
Professor caught this in Egypt… he was immunocompromised
had it in his bloodstream, not a single antibiotic could help because sugar capsule prevents access to bacteria
Ray Young used bacteriophages to treat him
bacteria cells very quickly mutate and change their sugar capsule to withstand bacteriophage attack… changed daily
antibiotics is class of biological molecules that can be incorporated in cell walls and prevent proliferation of bacteria… discovered from petri dish
penicillin discovered in ‘30s, used in WW2… more killed by wounds instead of bullets
put gunpowder in wounds… only way to disinfect
sepsis is when bacteria gets in bloodstream, rate or survival dec.
Sugars in Immune Response
Carbohydrates can be directly recognized by T cells or participate in T-cell stimulation as components of T-cell epitopes
carbs are key players in immune system
T-cell recognition of carbohydrate antigens takes place via their presentation by major histocompatibility complex pathways on antigen-presenting cells
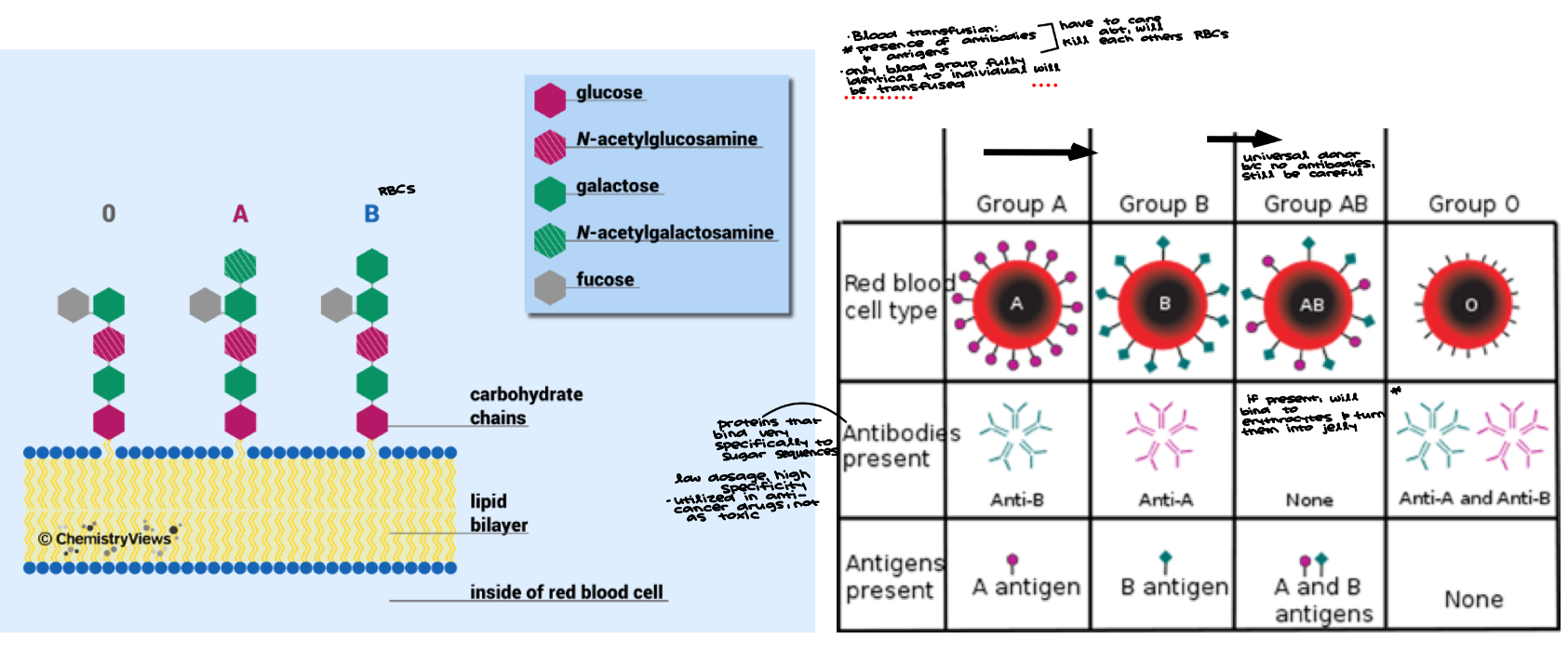
Blood and Carbohydrates ABO
Blood transfusion:
presence of antibodies and antigens (have to care about, will kill each others RBCs
only blood group fully identical to individual will be transfused
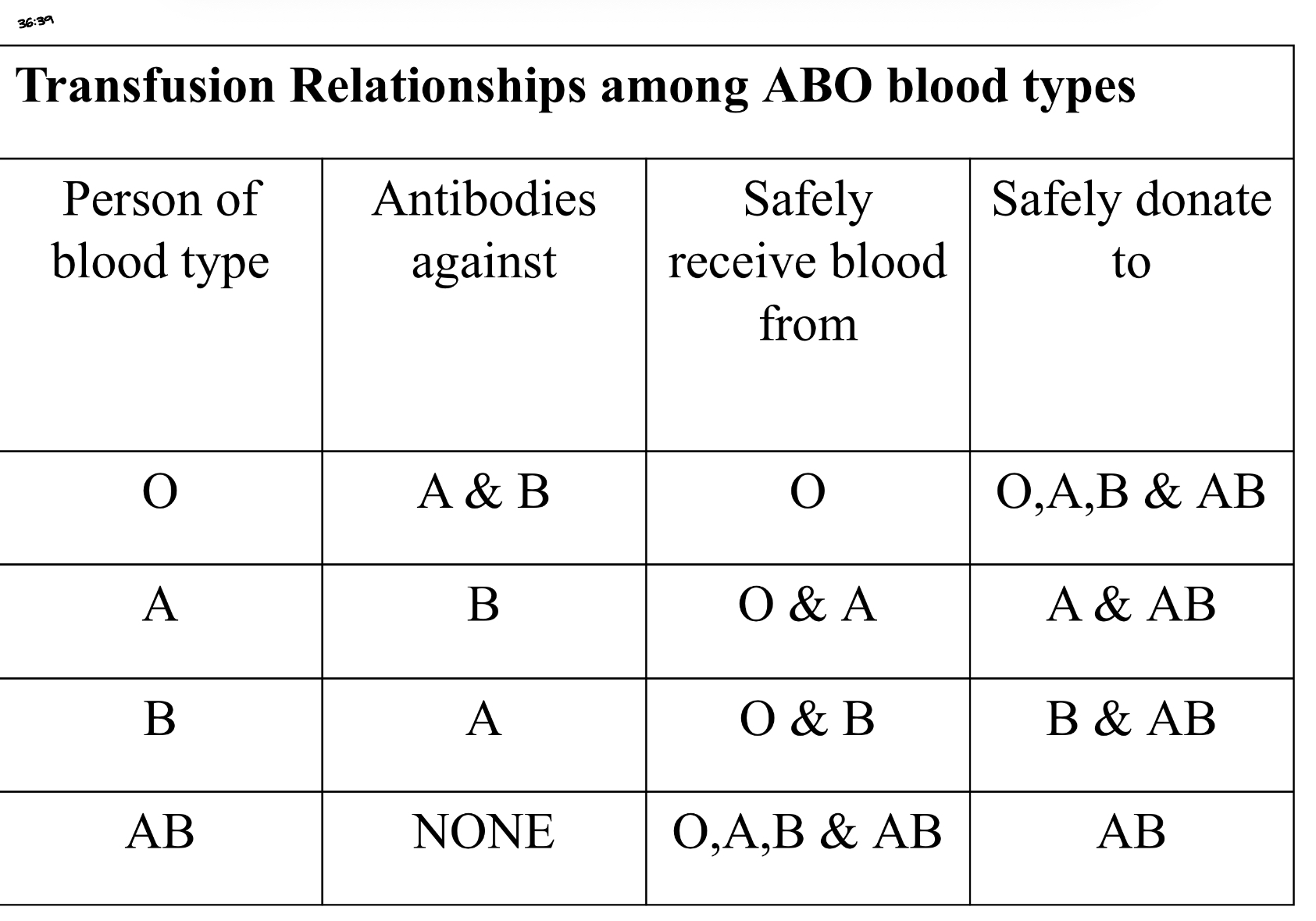
Transfusion Relationships among ABO blood types
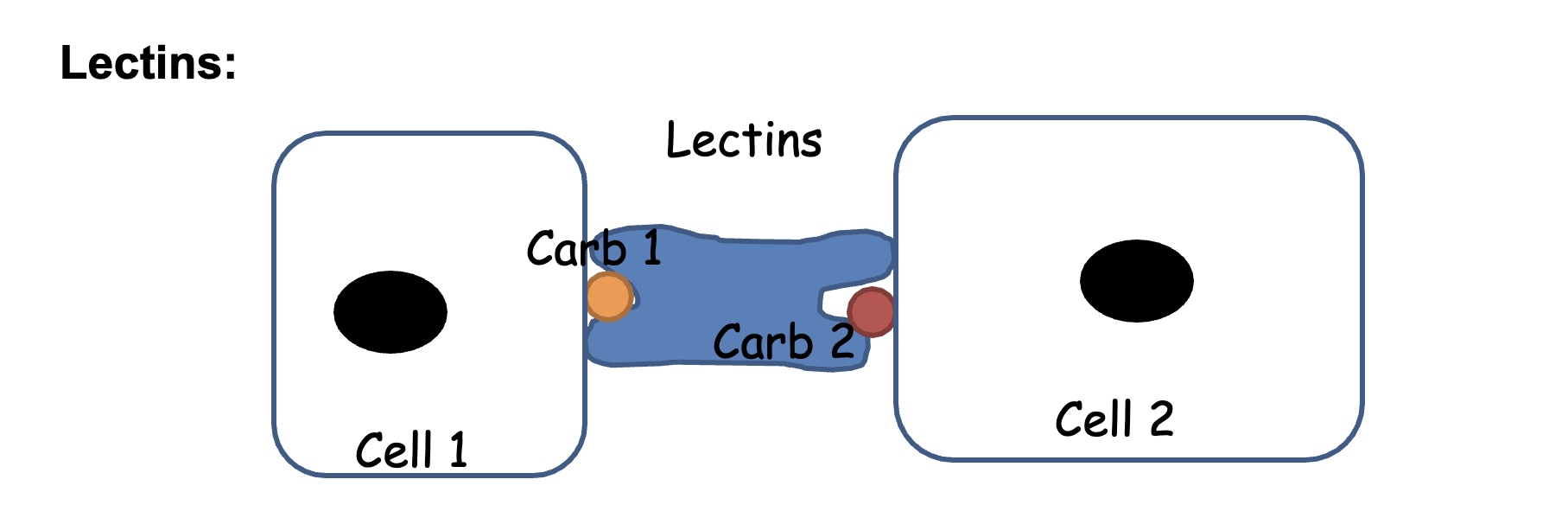
Cell-cell interaction
Selectin: transmembrane glycoproteins that mediate the attachment between leukocytes and epithelial cells. Recognize and bind specific oligosaccharides on cell-surface glycoproteins. Responsible for “capture of circulating leukocytes in blood.
lectins interact with sugars present in diff. cells
building blocks: allow cells in our bodies to stay integrated and form tissues
Virus-cell interactions
Sugars play important roles in recognition of viruses
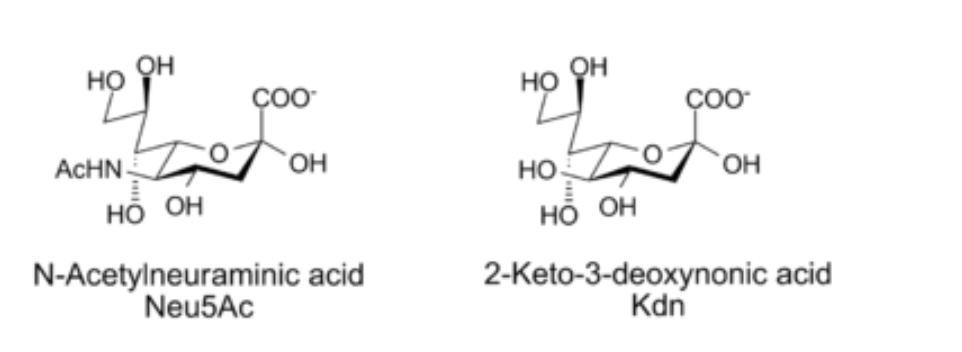
Influenza virus binds to sialic acid residues (Neu5Ac) that are present on cell surface glycoprotein
internalized by cells through endocytosis
cut off bond, proliferate towards nucleus where they inject their
After the virus penetrates the cell membrane, another viral protein, neuraminidase (sialidase), cleaves the glycosidic bonds to the sialic acid residues, freeing the virus to infect the cell. Inhibitors of this enzyme such as Tamiflu and Relenza are important anti-influenza agents
Glucose Regulation
High level of glucose in blood is dangerous. It is lowered through secretion of insulin by B cells of pancreas
Insulin promotes uptake of glucose by cells lowering its level in blood
Low levels of glucose are also dangerous, especially for neurons
Upon low glucose level in blood, glucagon is released which promotes hydrolysis of glycogen in liver
Helpful Study Hints
Look for commonalities in structures:
know glucose and galactose (linear to cyclic forms); recognize that lactose is a disaccharide composed of glucose and galactose have B (1 → 4) linkage
Chitin is aminated glucose polymers with B (1 → 4) linkage
a-amylose and cellulose are isomers
a-amylose has a (1 → 4) linkages
cellulose has B (1 → 4) linkage
Bioremediation
Jeff Fonda? uses soldier pliers (pliers that can consume a ton of biological material in a very short time frame) b/c they grow very quickly so people think that soldier pliers can be used to bioremediate dangerous wastes that are produced by humans
Trying to see if soldier pliers can be used to bioremediate prions
prion proteins will change structure of proteins… can self-assemble (very toxic) & cause many diseases in humans as well as in animals, including chronic waste disease
If you hunt, do not eat the brain or lymph nodes of animals
if blood of deer that has prions drops on a plant, plant will possess prions and if next year we consume plant, we will be impacted by prions
prions can stay on plants for years, they are very stable structures
One of many problems we try to address in the state of Texas is screwworm fly
was in Texas in 70s and 80s, can lay 200-300 eggs on fresh wounds (infestations)
larvae that hatch from these eggs will burrow inside tissue and will develop in animals
at late stages, become big (3-4x bigger than grain of rice)
causes cow death in 5-7 days
nuclear rxn… fly can go around 12 miles, how it spreads
Aggie professor proposed to grow and cultivate flied and x-ray larvaes. Flies that hatch from this larvae will be sterile… will mate with wild flies, but produce no fertile eggs. Approach worked and problem was pushed away from Texas. U.S. had partnership with Panama and with war in South Panama… all flied got pushed there. What took 18 years to do was back in 2 days because of COVID. Fly back in TX, people are losing cattle
monitor cattle and diff. size larvae