Chapter 12 --> Solids and modern materials
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
describe a crystalline solid.
when allowed to cool slowly, the particles in a liquid will arrange themselves to give the maximum attractive forces; resulting in a crystalline solid
what is the arrangement of the particles in a crystalline solid called?
crystal lattice
What is the unit cell of the particles?
the smallest unit that shows the pattern of arrangement
describe unit cells.
three dimensional, repeated over and over to give macroscopic crystal structure
what is each particle in the unit cell called?
lattice point
what is a coordination number?
the number of other particles each particle is in contact with
higher coordination number means _________; therefore, __________________ hold the crystal together
more interaction; stronger attractive forces
what is packing efficiency?
percentage of volume in the unit cell occupied by particles
describe a cubic unit cell.
all 90 degree angles at the corners, the length of all edges are equal, if spherical particles: 1/8 of each corner is within the cube, 1/2 of each face is within the cube, and 1/4 of each edge is within the cube
what is the coordination number of a simple cubic cell?
6
how many atoms are in a simple cubic cells?
1
Simple cubic
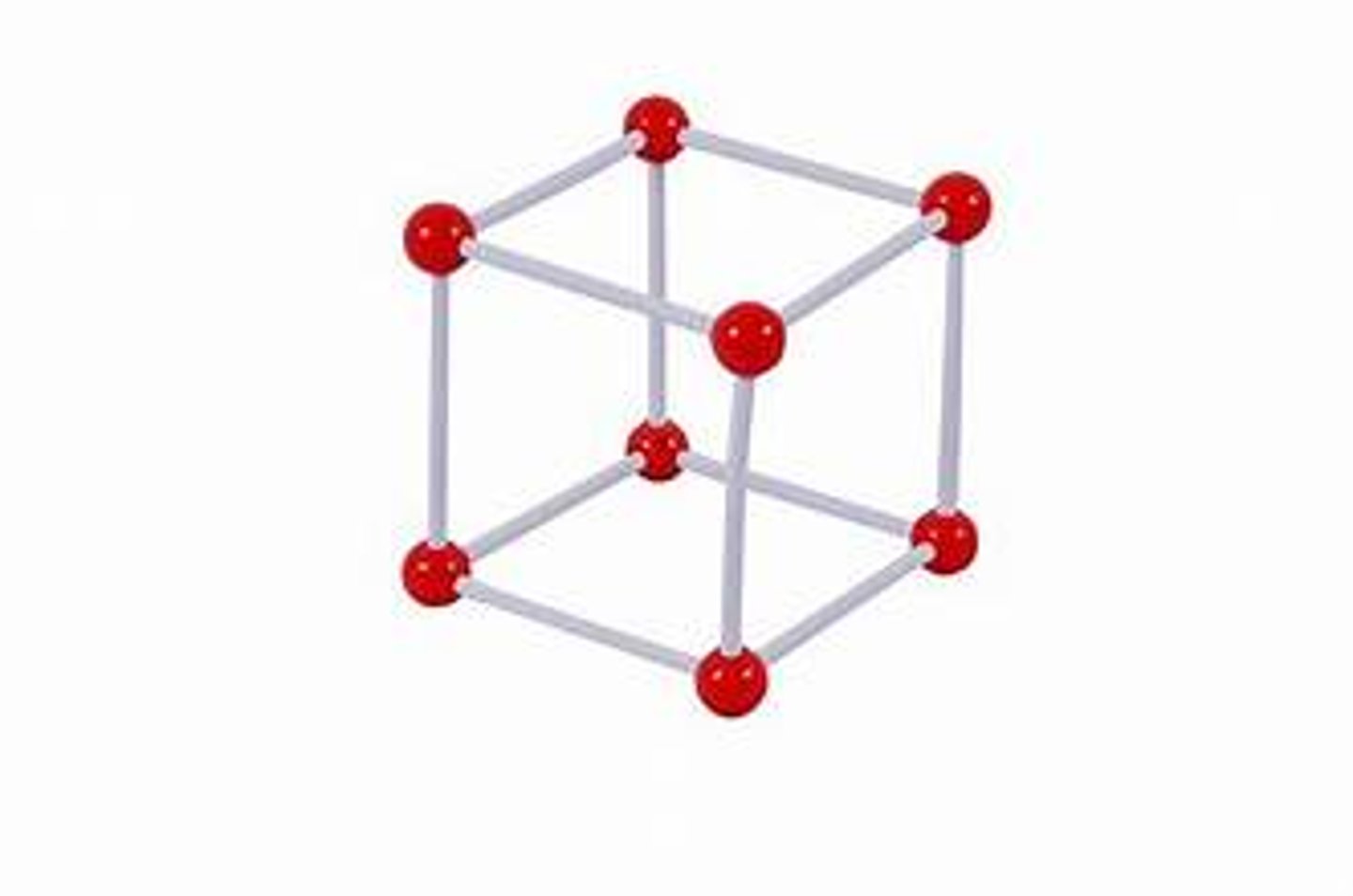
what is the coordination number of a body centered cubic cell?
8
how many atoms are in a body-centered cubic cell?
2
body centered cubic
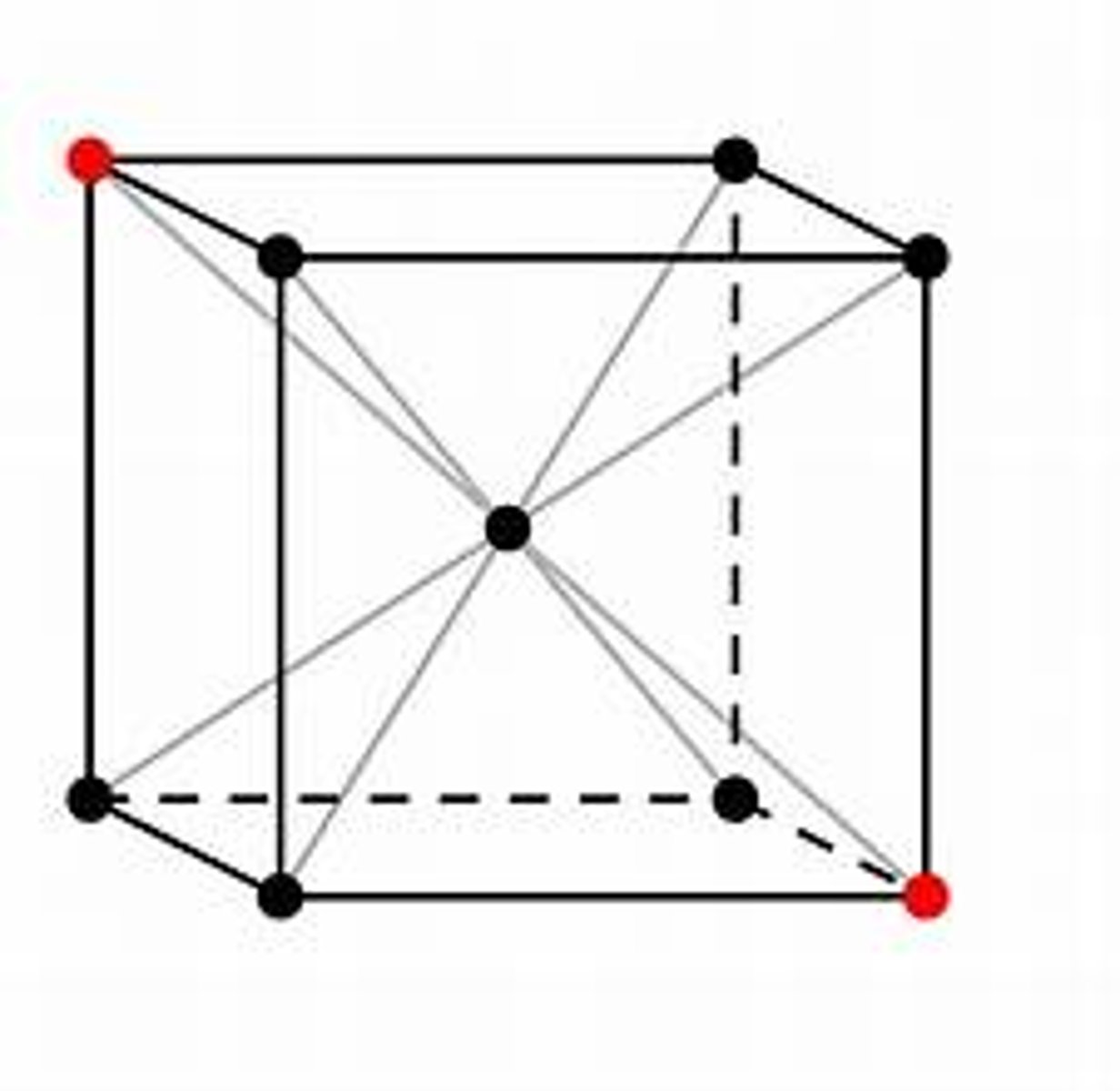
what is the coordination number of a face-centered cubic cell?
12
how many atoms are in a face-centered cubic cell?
4
face centered cubic
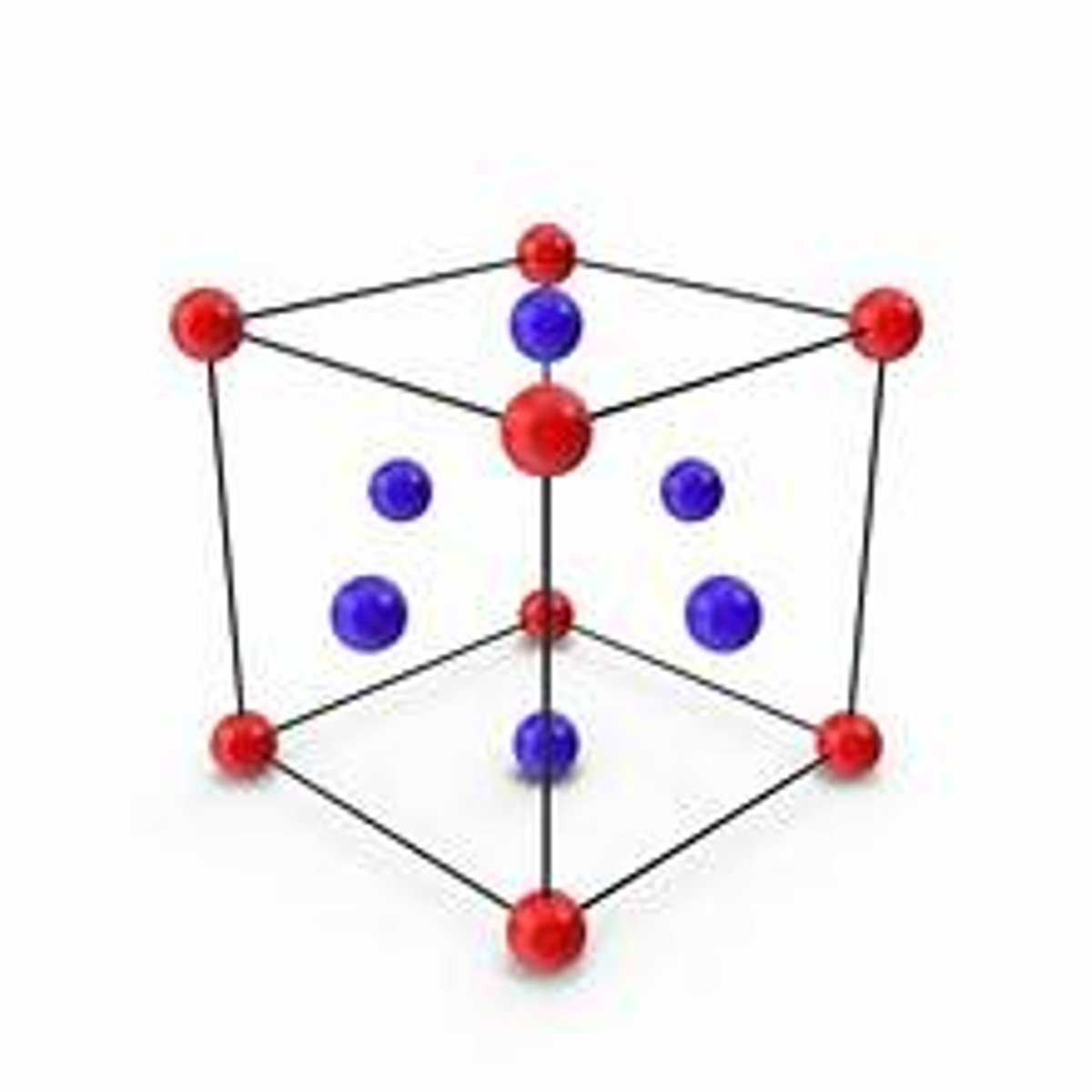
describe polymers.
many small units called monomers that link together
give some characteristics of polymers.
chain lengths can be varying, results in a mixture, very large molar masses
Longer chains = ______________
stronger polymers
polar side groups give stronger attraction between polymer chains, making the polymer _________
stronger
straight, unbranched chains can pack together more closely, giving that polymers have a ___________ and therefore _________________
higher density; stronger attraction
if polymer chains are linker together extensively by covalent bonds, the polymer is harder and ______________________________________
more difficult to melt