C5.1 Exothermic and endothermic reactions
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
What is an exothermic reaction + examples?
A chemical reaction that transfers energy to its surroundings, leading to an increase in temperature of the surroundings
Examples: combustion, respiration
What is an endothermic reaction + examples?
A chemical reaction that absorbs energy from its surroundings, leading to a decrease in temperature of the surroundings
Examples: photosynthesis, melting ice
In which reaction are the products more stable and in which are the reactants more stable?
Reactants are more stable in endothermic reactions
Products are more stable exothermic reactions
What is the transfer of thermal energy during a reaction called?
Enthalpy change, represented by ∆H
In exothermic reactions: -∆H
In endothermic reactions: ∆H
What is activation energy (Ea)?
The minimum energy colliding particles must have to react
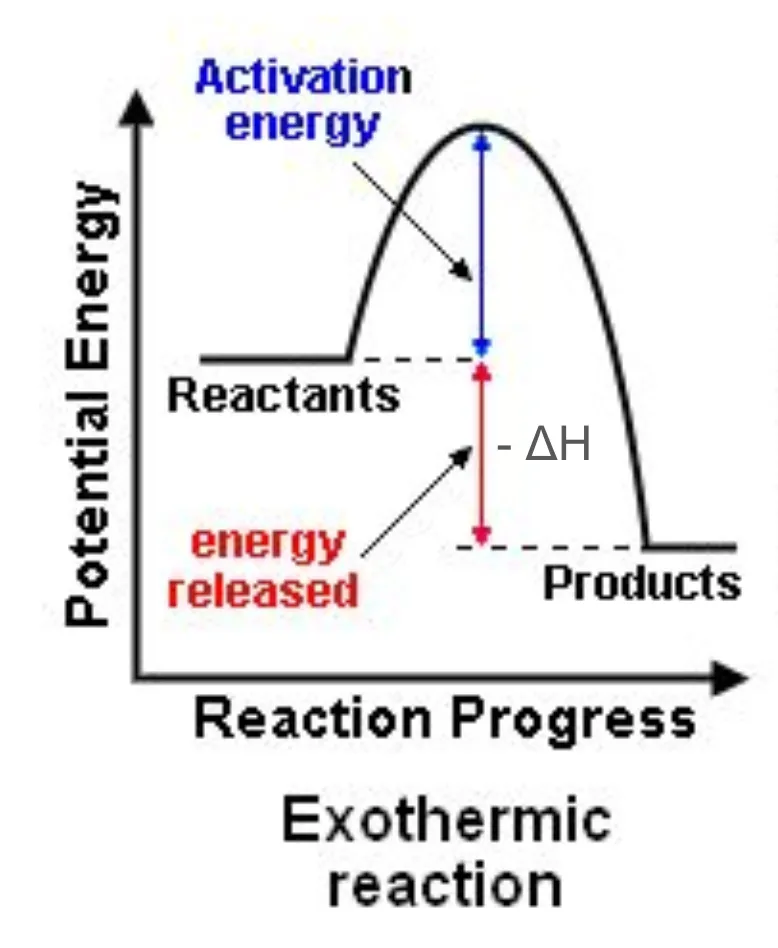
Draw and label a reaction pathway diagram for exothermic reactions
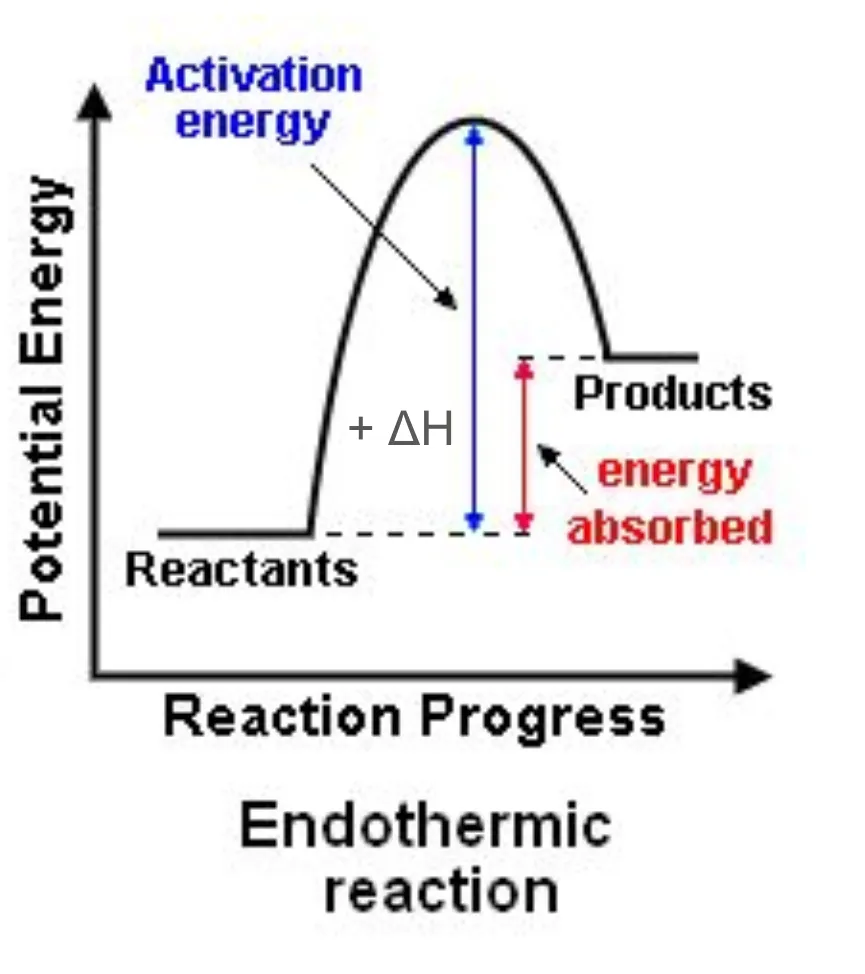
Draw and label a reaction pathway diagram for endothermic reactions
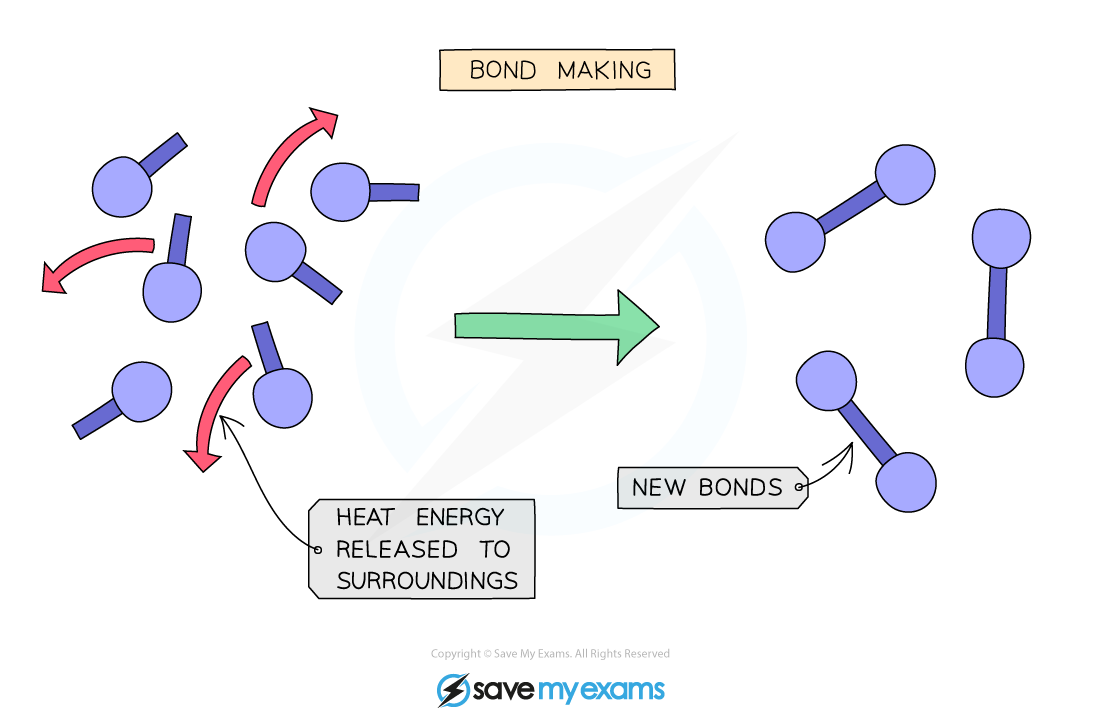
What type of reaction is bond making?
Bond making: exothermic process
Bonds release energy into surroundings to achieve a stable configuration
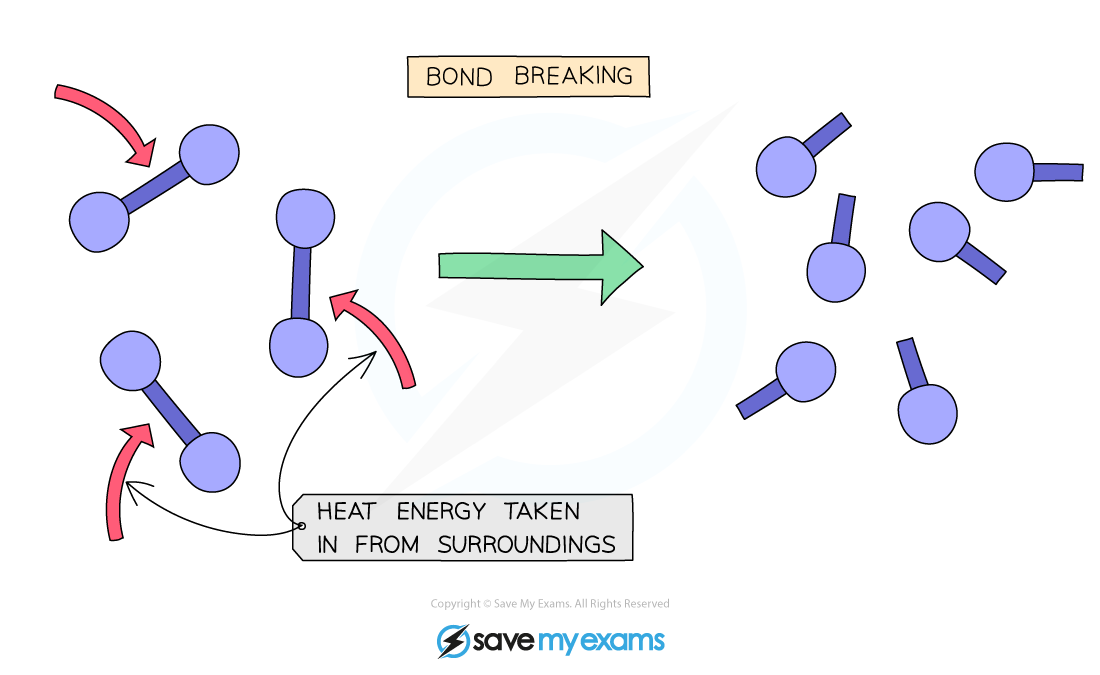
What type of reaction is bond breaking?
Bond breaking: endothermic process
Bonds absorb energy to be able to have enough to break apart atoms held together by intermolecular forces