TLC of Photosynthetic Pigments 🍂
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
14 Terms
What is the purpose of thin-layer chromatography (TLC) for pigments?
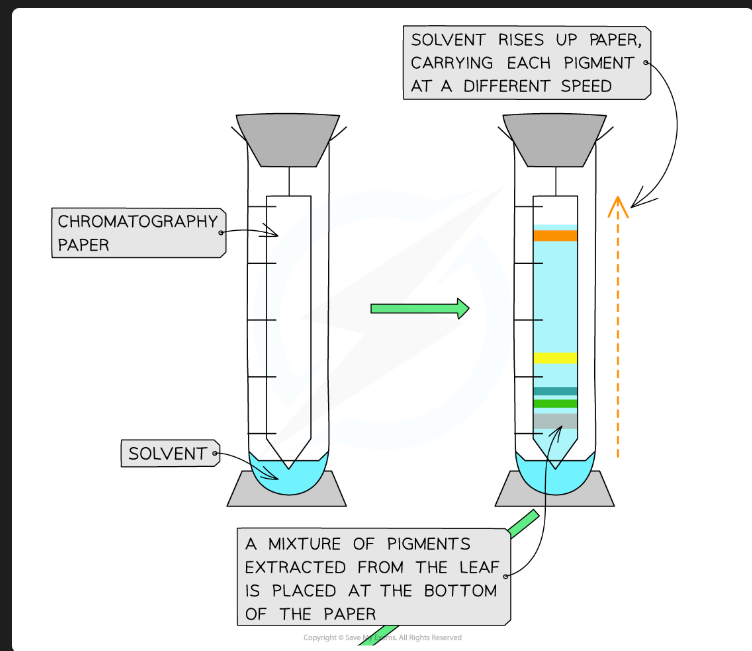
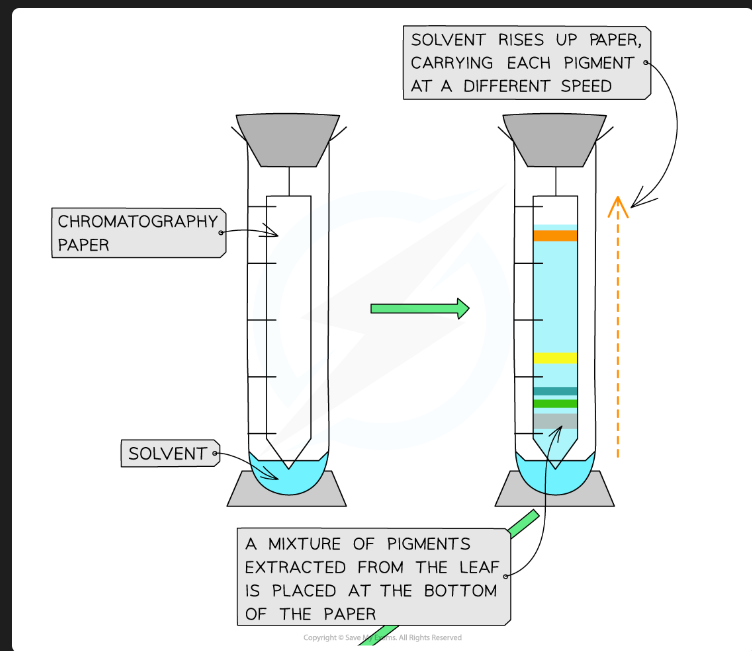
To separate the different photosynthetic pigments (e.g., chlorophyll a, chlorophyll b, carotenoids) from a leaf extract.
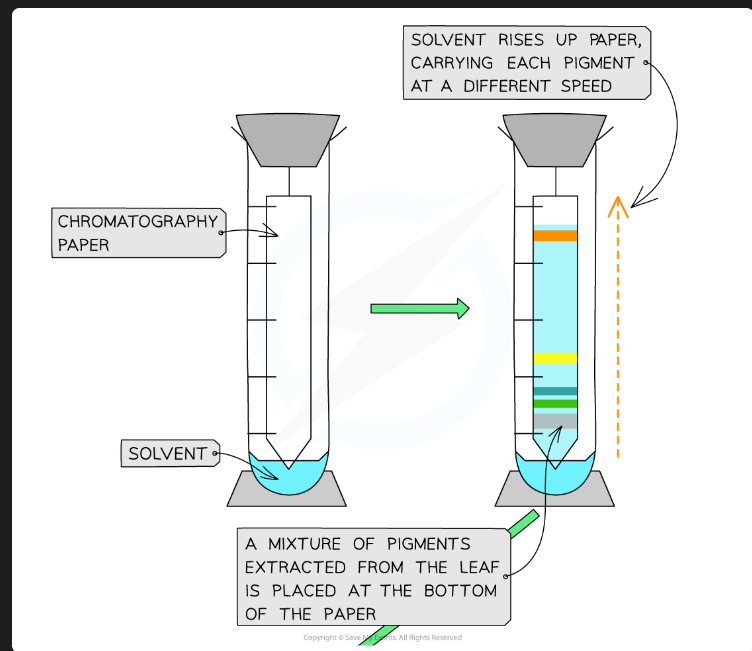
What are the stationary phase and mobile phase in this practical?
Stationary Phase: The TLC plate (coated in silica gel or alumina).
Mobile Phase: The solvent (e.g., a mixture of organic solvents).
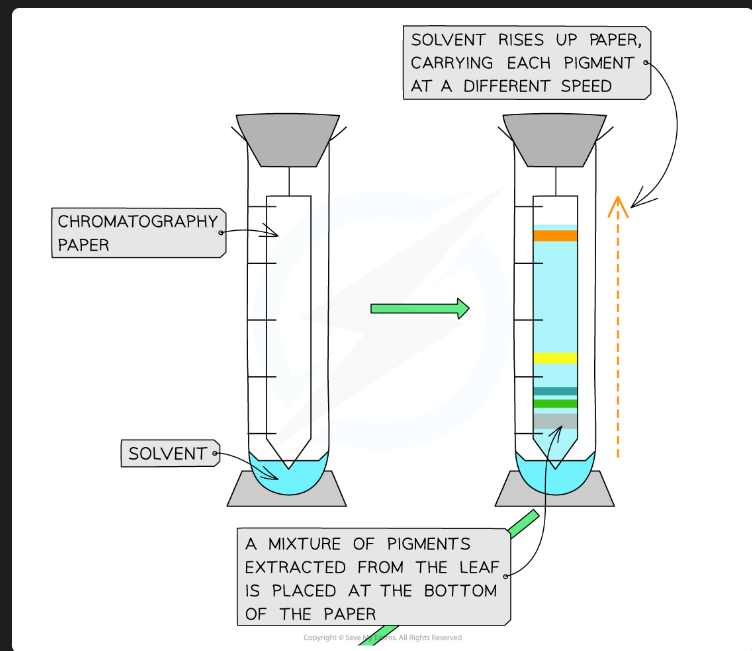
On what principle does separation by TLC depend?
Separation is based on the solubility of the pigment in the mobile phase vs. its affinity/adsorption to the stationary phase.
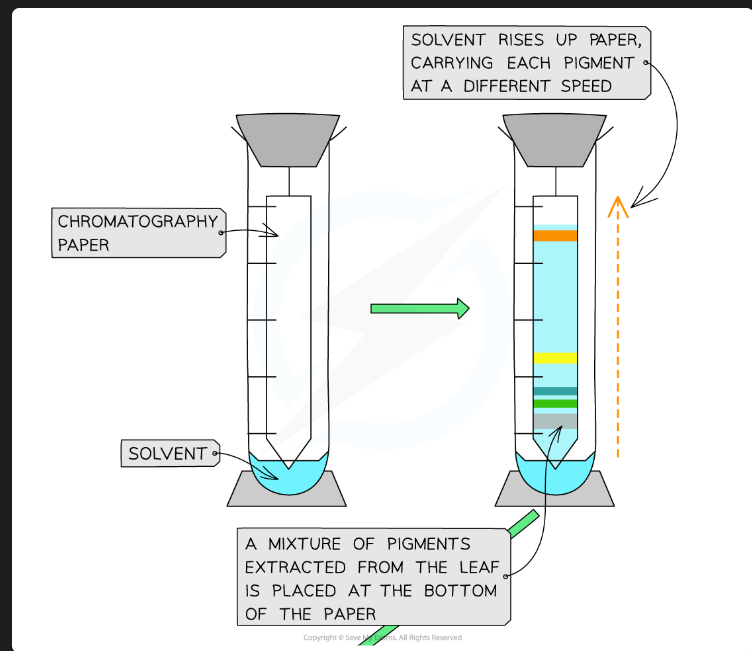
Which pigments travel furthest up the plate?
The pigments that are most soluble in the mobile phase and have the lowest affinity (are least strongly adsorbed) to the stationary phase.
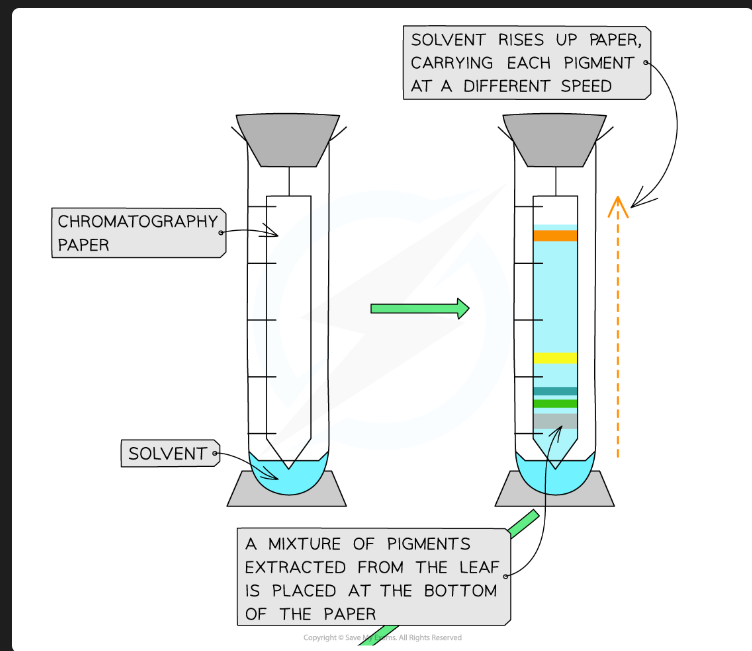
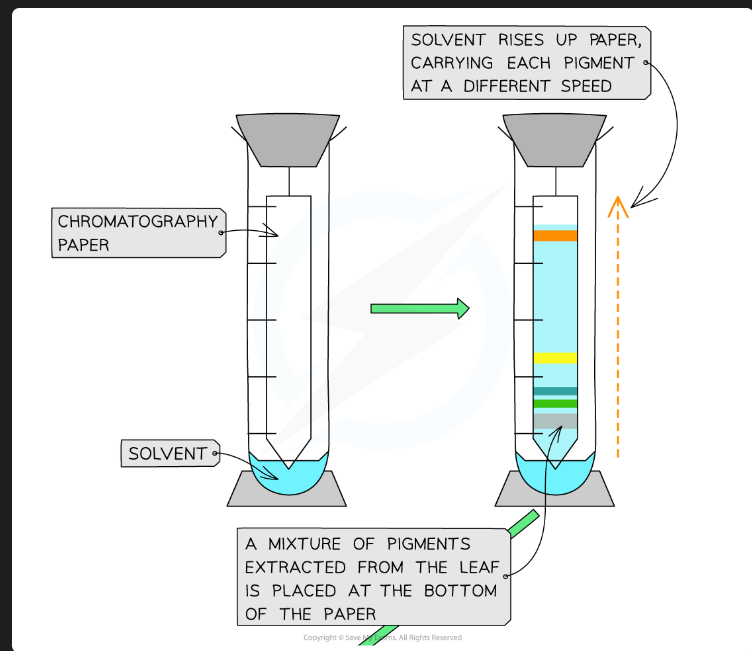
Why must the origin line be drawn in pencil?
Pencil is insoluble in the solvent. If you used an ink pen, the ink pigments would dissolve and separate along with the plant pigments, invalidating the results.
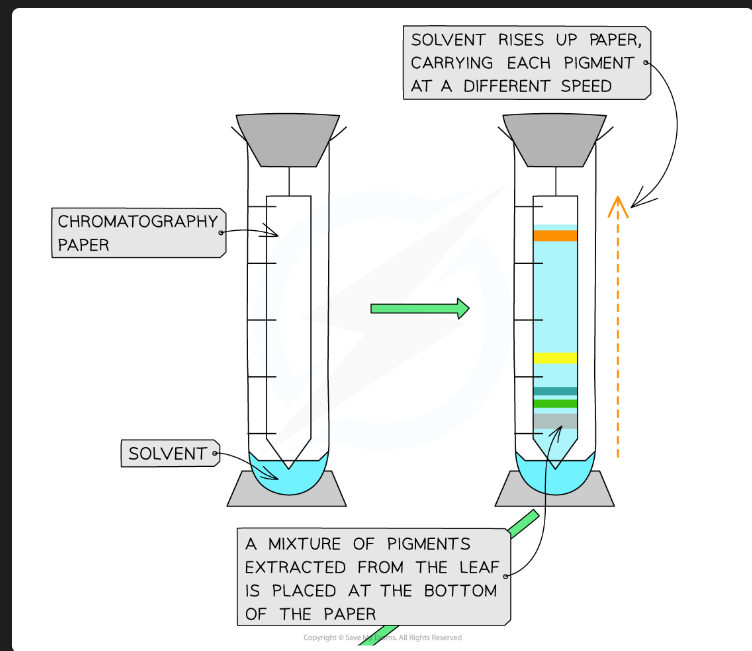
Why must the solvent level in the beaker be below the origin line?
If the solvent is above the origin, the pigment spot will just dissolve into the reservoir of solvent at the bottom instead of being carried up the plate.
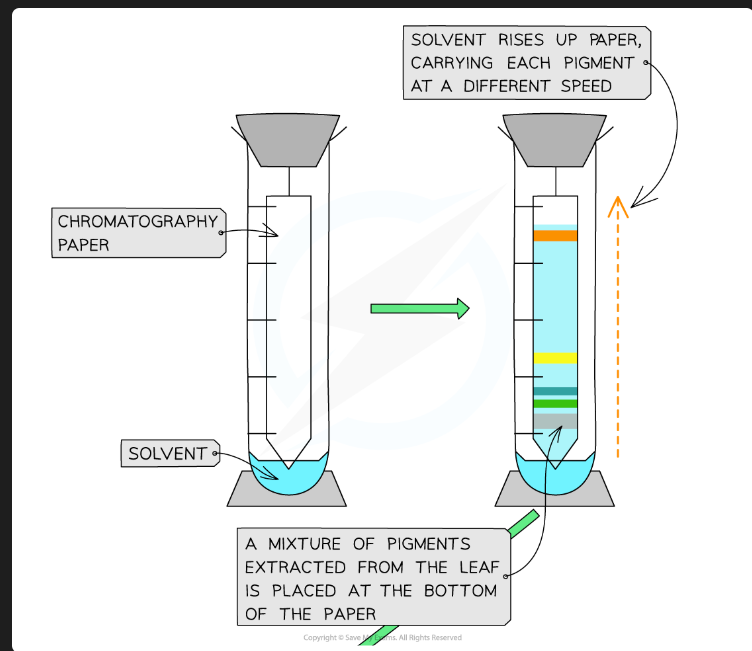
Why should you put a lid on the beaker?
To create a saturated atmosphere (the air is saturated with solvent vapour). This prevents the solvent from evaporating from the surface of the plate as it moves up, which ensures an even run.
How do you calculate the Rf value (retardation factor)?
R_f = \frac{\text{Distance moved by pigment spot}}{\text{Distance moved by solvent front}}
An R value is always...
...less than 1. (Because the solvent front always moves further than any pigment spot).
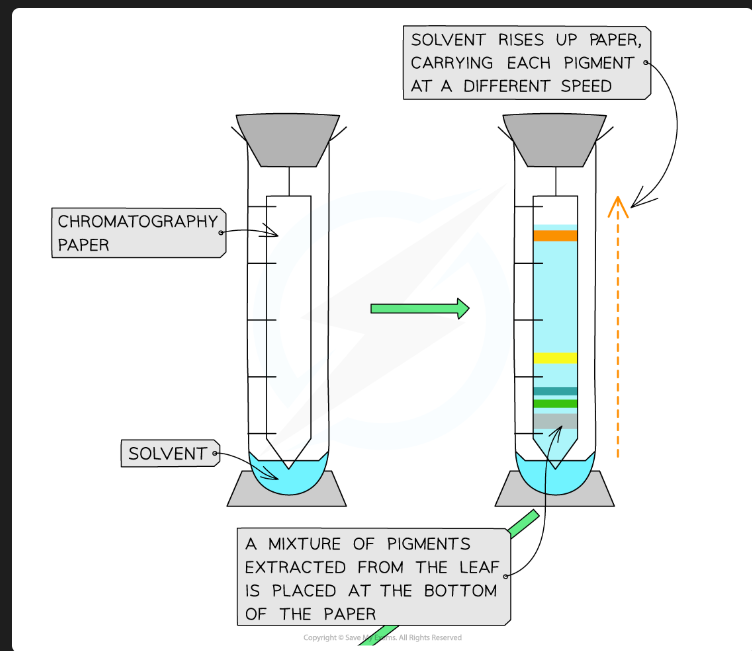
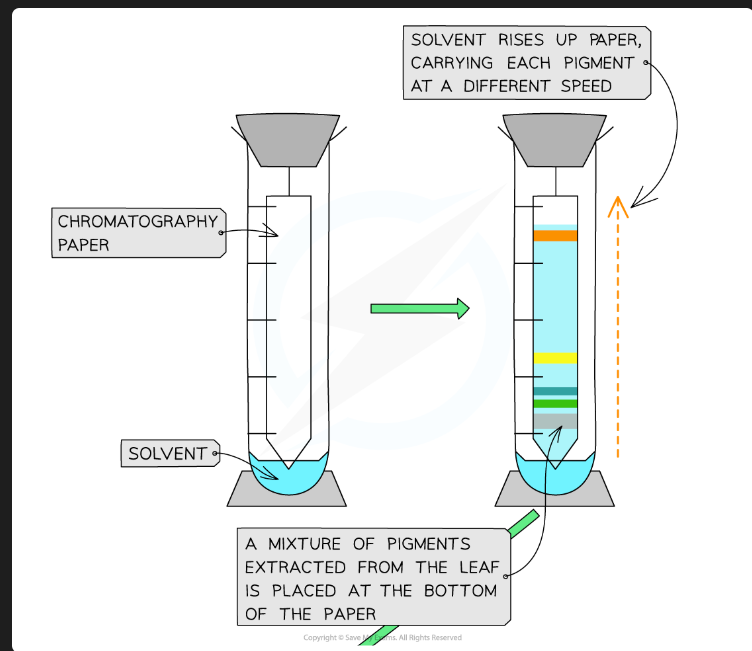
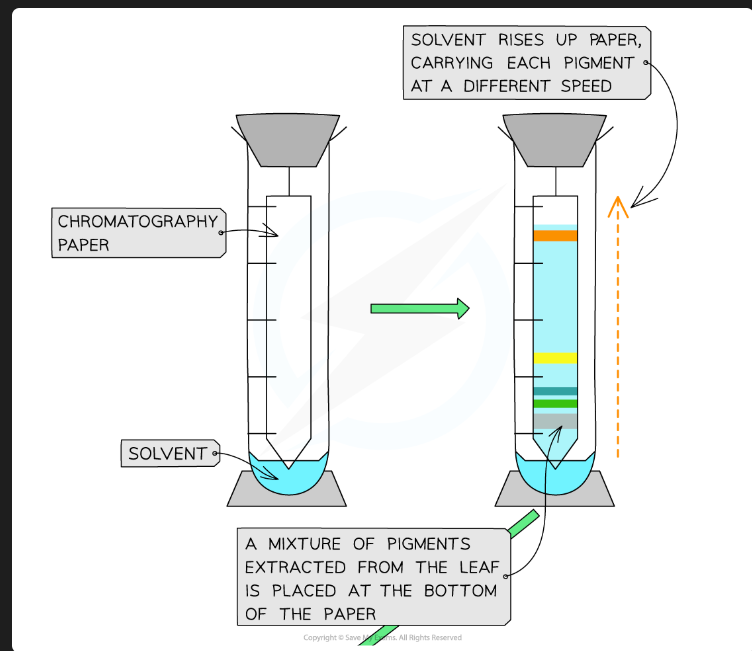
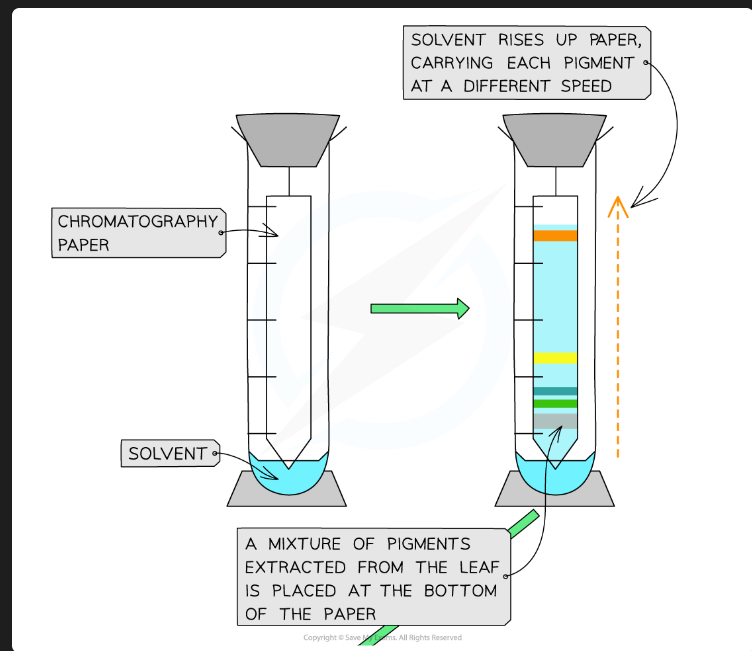
What pigments would you expect to see, in order from top to bottom?
Carotenes (e.g., Beta-carotene - orange)
Xanthophylls (yellow)
Chlorophyll a (blue-green)
Chlorophyll b (yellow-green)
(Chlorophyll b is the most polar/least soluble, so it's at the bottom).
Describe a method to separate photosynthetic pigments from a leaf using thin-layer chromatography (TLC).(6 marks)
Extract: Grind a leaf with a solvent (e.g., propanone) and sand (using a pestle and mortar).
Origin: Draw a pencil line (origin) near the bottom of a TLC plate.
Spot: Use a capillary tube to build up a small, concentrated spot of the pigment extract on the origin.
Chamber: Place the plate in a beaker with a small amount of solvent (mobile phase), ensuring the solvent level is below the origin line.
Run: Cover the beaker (e.g., with a watch glass) and allow the solvent to run up the plate.
Finish: Remove the plate when the solvent front is near the top and immediately mark its position in pencil.
Analyse: Calculate Rf values for each separated spot.
In a TLC experiment, the solvent front moved 9.2 cm from the origin. A yellow pigment spot moved 6.8 cm from the origin. Calculate the Rf value for this pigment. (Show your working).

Front: Explain the principle of separation in TLC. Why do different pigments travel at different speeds?
The principle involves two phases: a stationary phase (the TLC plate) and a mobile phase (the solvent).
Pigments have different solubilities in the mobile phase and different affinities (adsorption) for the stationary phase.
A pigment that is more soluble in the solvent and has a lower affinity for the plate will be carried further up.
A pigment that is less soluble and has a higher affinity for the plate will travel slower and not as far.
A student failed to put a lid on the developing tank. Explain what effect this would have on the Rf values.
Without a lid, the solvent will evaporate from the surface of the plate as it moves up.
This will slow down the movement of the solvent front, so the solvent front will not travel as far as it should.
This would lead to higher (and inaccurate) Rf values, as the denominator (distance moved by solvent front) would be artificially small.