MCAT -- Amino Acids + Proteins (High Yield)
1/99
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
100 Terms
What are amino acids?
building blocks of proteins
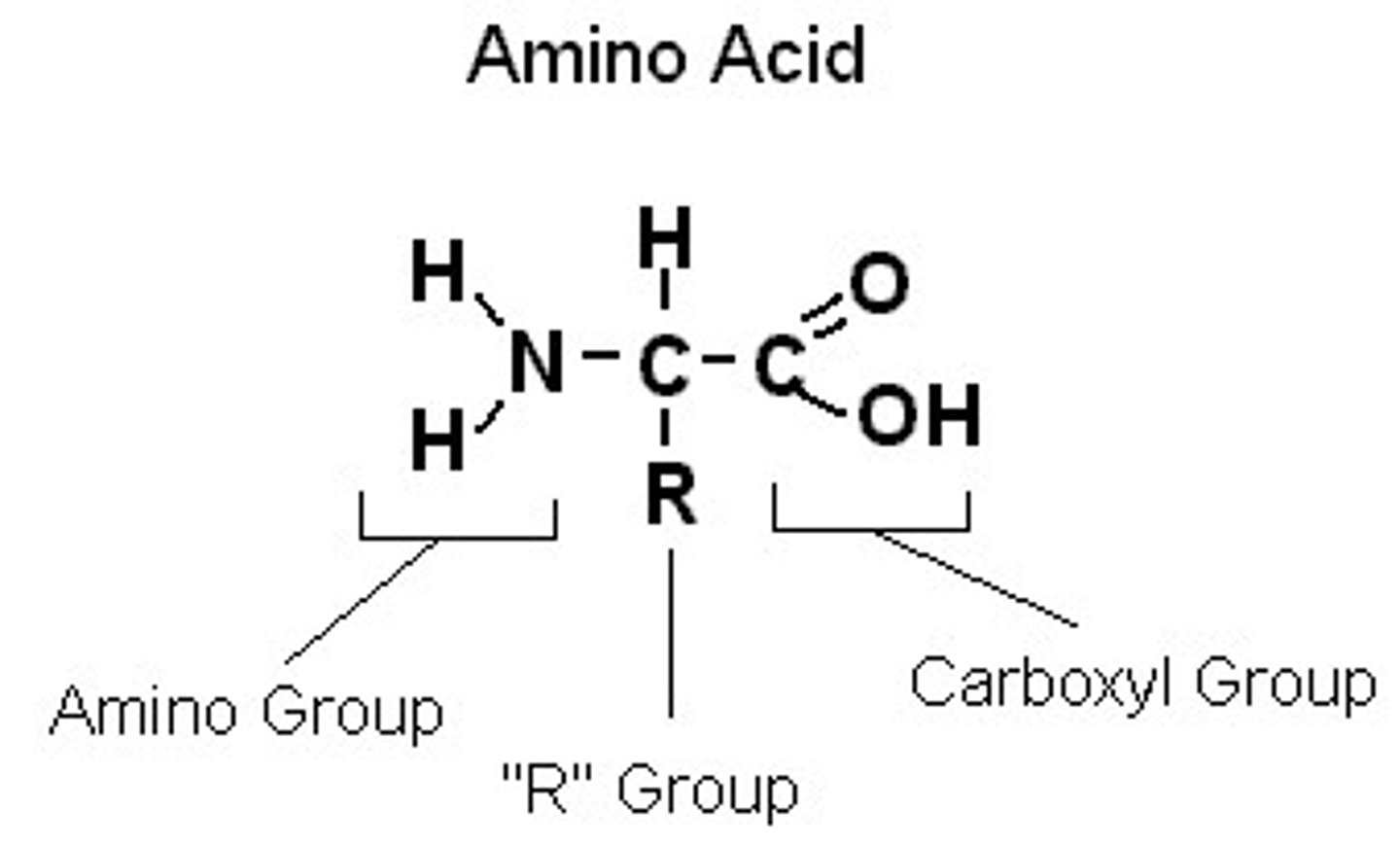
What is the structure of amino acids?
molecules that contain two functional groups: an amino group (-NH2 ) and a carboxyl group (-COOH).
What are α-amino acids?
amino acids in which the amino group and the carboxyl group are bonded to the same carbon: the α-carbon of the carboxylic acid.
How can we determine where the alpha carbon is?
the α-carbon is the central carbon of the amino acid; for carboxylic acids, the α-carbon is the carbon adjacent to the carboxyl carbon. A carboxylic acid is an organic acid that contains a carboxyl group (C(=O)OH) attached to an R-group. The general formula of a carboxylic acid is R−COOH. Thus, an amino acid with a carboxyl functional group and a side group contain carboxylic acids.
What groups are attached to the alpha carbon?
amino and carboxyl groups, a hydrogen atom, and a side chain, also called an R group (4 groups in total).
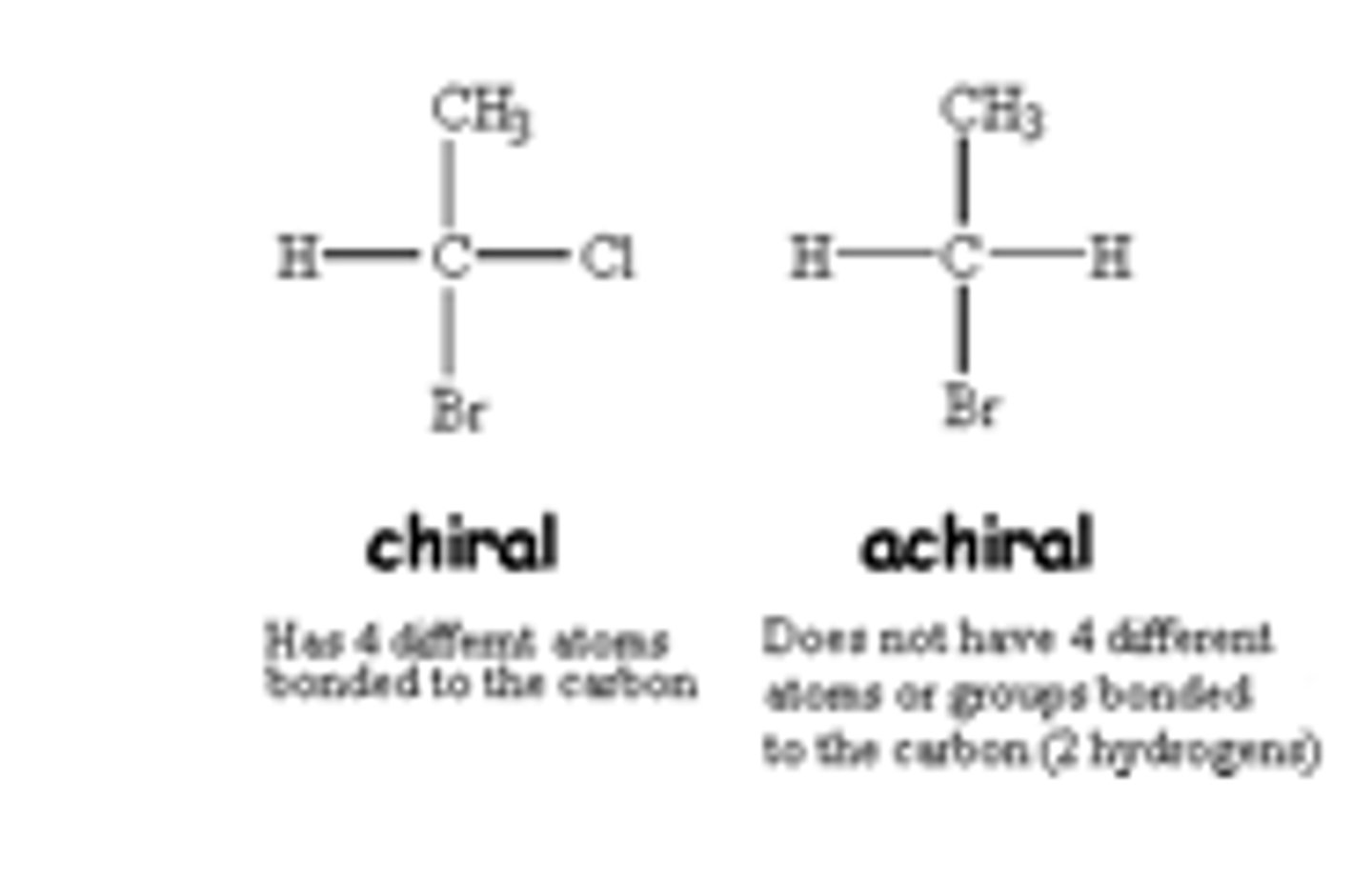
For most amino acids, the α-carbon is a ________________ center, as it has four different groups attached to it; The one exception is glycine, which has a hydrogen atom as its R group, making it _____________.
chiral (or stereogenic); achiral.
What are chiral centers?
Chiral centers are tetrahedral atoms (usually carbons) that have four different substituents.
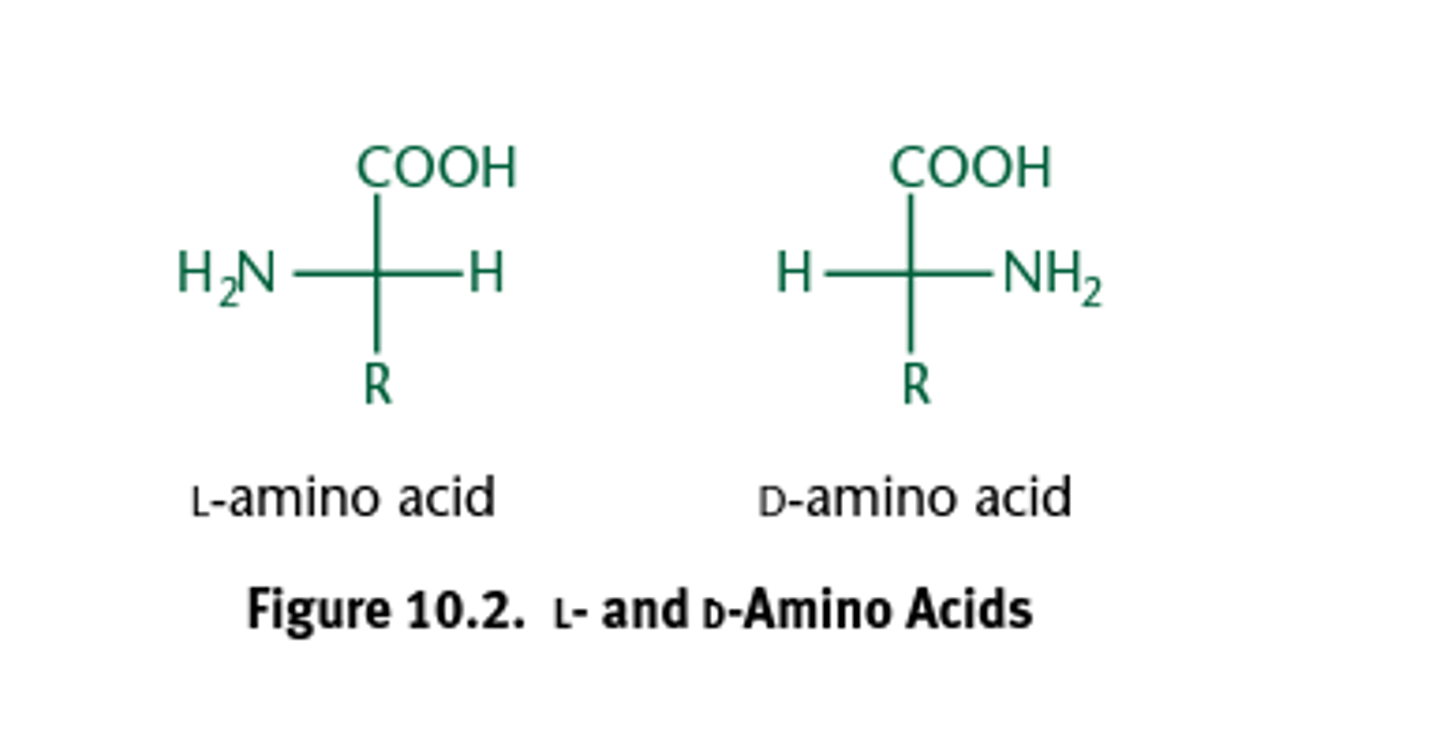
All chiral amino acids used in eukaryotes are _____________.
L-amino acids.
All naturally occurring amino acids in eukaryotes—except for glycine—are optically active, and all are L-isomers.
What about glycine? Glycine is the only amino acid which does not have a chiral carbon atom, so it does not form stereoisomers therefore will not have L or D configurations.
Except for __________, all L-amino acids have an "___" absolute configuration (its stereochemistry).
cysteine; "S".
Cysteine has an "_____" absolute configuration.
R
T/F: The 20 eukaryotic proteogenic amino acids are grouped into five categories.
True.
Nonpolar nonaromatic, aromatic, polar, negatively charged (acidic), and positively charged (basic).
What are the amino acids with non-polar, non-aromatic side-chains?
Amino acids with non-polar, non-aromatic side chains are: glycine (Gly; G), alanine (Ala; A), valine (Val; V), leucine (Leu; L), isoleucine (Ile; I), methionine (Met; M), and proline (Pro; P).
ACRONYM: GAVLIMP -- Grandma Always Visits London In My Pajamas.
What determines whether an amino acid is aromatic or not?
Three rules of aromaticity:
1.) They are rings
2.) They must be planar
3.) The ring must follow Huckel's rule.
Acronym to remember the three aromatic amino acids: WFY -- you put a RING on a soon-to-be WIFEY!
There are ________ aromatic amino acids that are considered proteinogenic.
3
What are the amino acids with uncharged, aromatic side-chains?
tryptophan (Trp; W), phenylalanine (Phe; F), tyrosine (Tyr; Y).
ACRONYM: WFY (can think of as spelling WIFEY).
What are the amino acids with polar side-chains?
serine (Ser; S), threonine (Thr; T), asparagine (Asn; N), glutamine (Gln; Q), cysteine (which has a thiol group in its side chain) (Cys; C).
ACRONYM: STNQC -- Steven Threaded Nine Quilted Caps.
Only ______ of the 20 amino acids have negative charges on their side chains at physiological pH (7.4).
two
What amino acids have negative charges on their side chains at a pH of 7.4?
aspartic acid (aspartate --Asp; D) and glutamic acid (glutamate -- Glu, E).
What are the amino acids with positively charged (basic) side chains at the physiological pH?
Lysine (Lys; K), arginine (Arg; R), and histidine (His; H).
ACRONYM for electrically charged side chains: DEKRH -- Dragons Eat Knights Riding Horses.
What are the hydrophobic amino acids?
alanine, valine, leucine, isoleucine, phenylalanine -- these amino acids have long alkyl side chains. Since they're strongly hydrophobic, more likely to be found in the interior of a protein (away from water on the surface of protein).
AVLIF -- A Valuable Lipid Is Fatty
What are the hydrophilic amino acids?
all amino acids with charged side-chains (positively and negatively): Lysine, arginine, histidine, glutamate and aspartate, and the amides: asparagine and glutamine.
What are the amide amino acids?
Asparagine (Asn, N) and Glutamine (Gln, Q). Named because they have an amide functional group.
T/F: The remaining amino acids lie somewhere in the middle and are neither particularly hydrophilic nor particularly hydrophobic.
True.
proteinogenic amino acids
the 20 alpha amino acids encoded by the human genetic code
When viewing the structures of amino acids, why do we see COO- instead of the normal carboxyl group, or NH3+ instead of the normal amino group?
Amino acids have a central asymmetric carbon to which an amino group, a carboxyl group, a hydrogen atom, and a side chain (R group) are attached. This amino acid is unionized, but if it were placed in water at pH 7, its amino group would pick up another hydrogen and a positive charge, and the hydroxyl in its carboxyl group would lose and a hydrogen and gain a negative charge.
In the aqueous environment of the cell, the both the amino group and the carboxyl group are ionized under physiological conditions, and so have the structures -NH3+ and -COO-, respectively.
Structure of Glycine
Gly, G
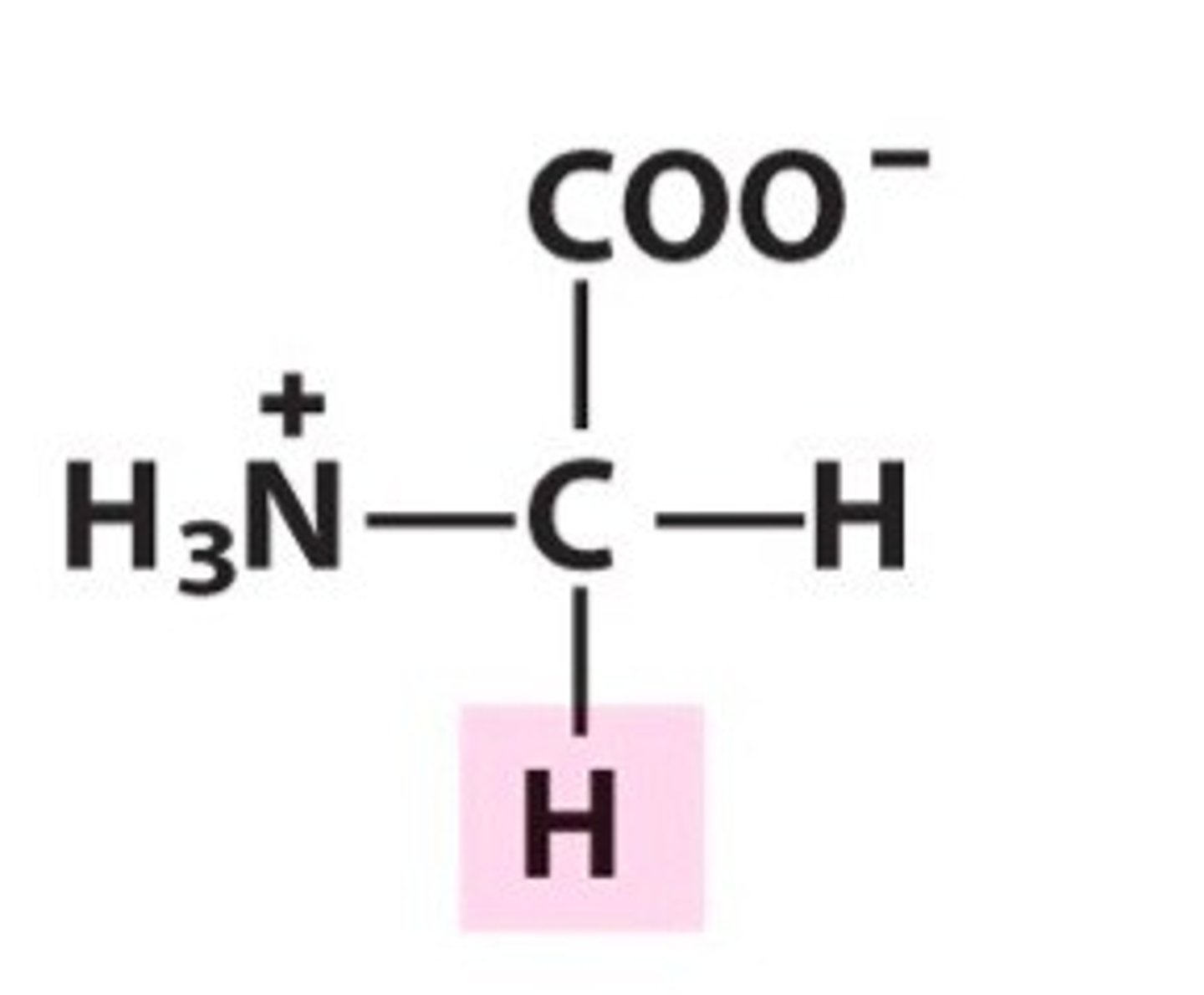
Structure of Alanine
Ala, A. Methyl group.
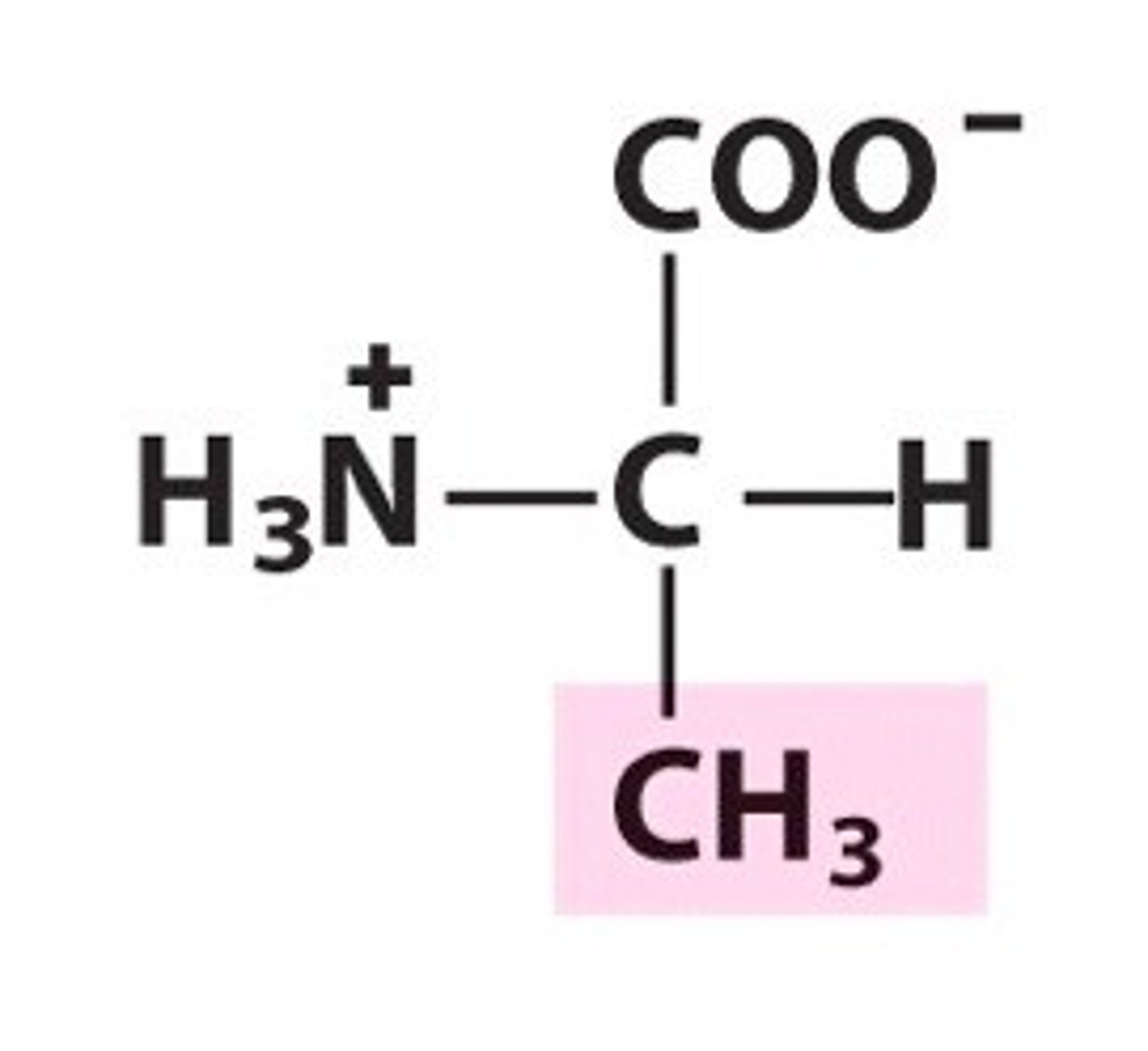
Structure of Valine
Val, V; can envision as a glycine where "H" R group is replaced by an isopropyl group (CH3-CH-CH3).
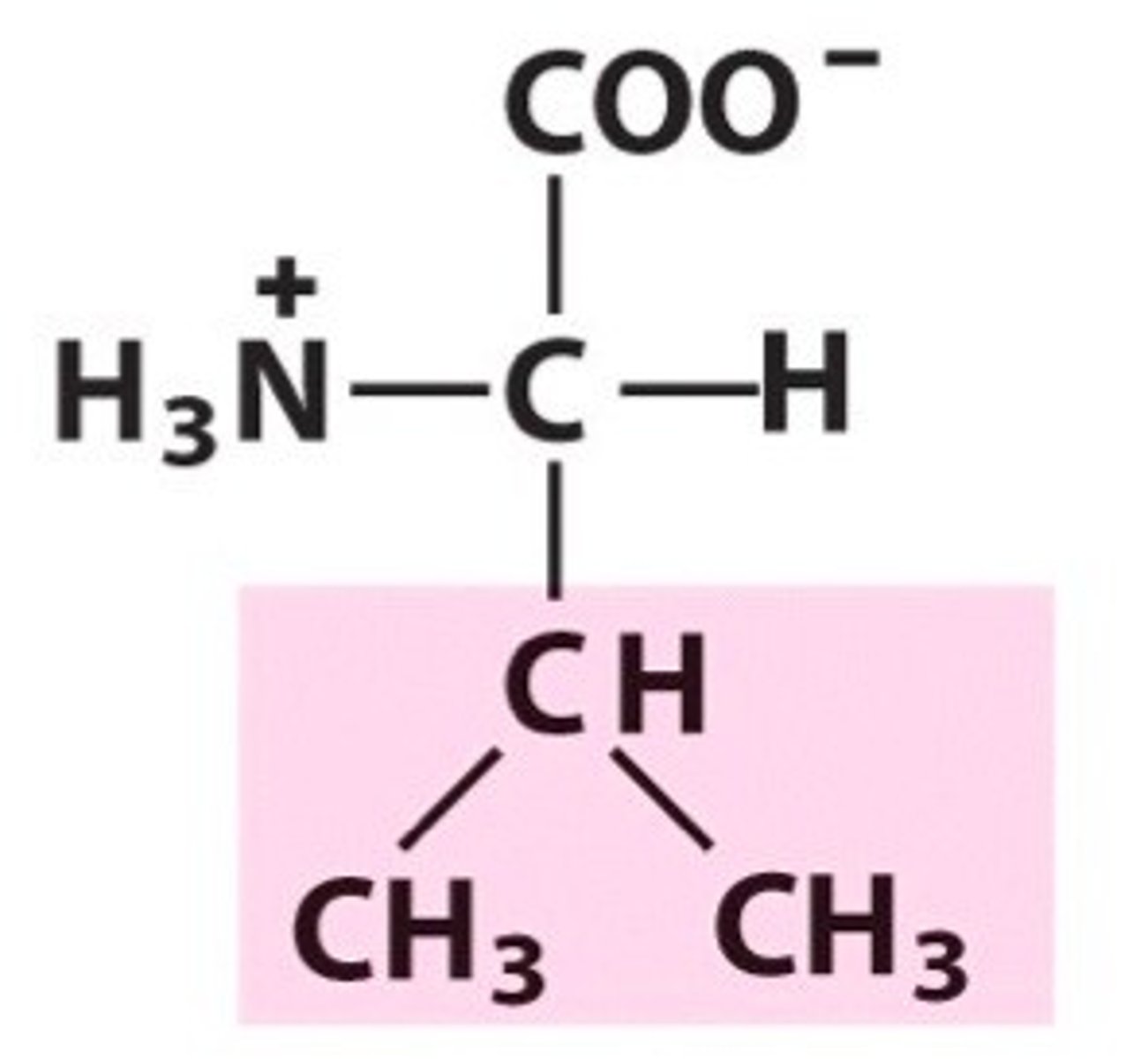
Structure of Leucine
Leu, L; can envision as a glycine where "H" R group is replaced by an isobutyl group (CH3-CH-CH2
|
CH3).
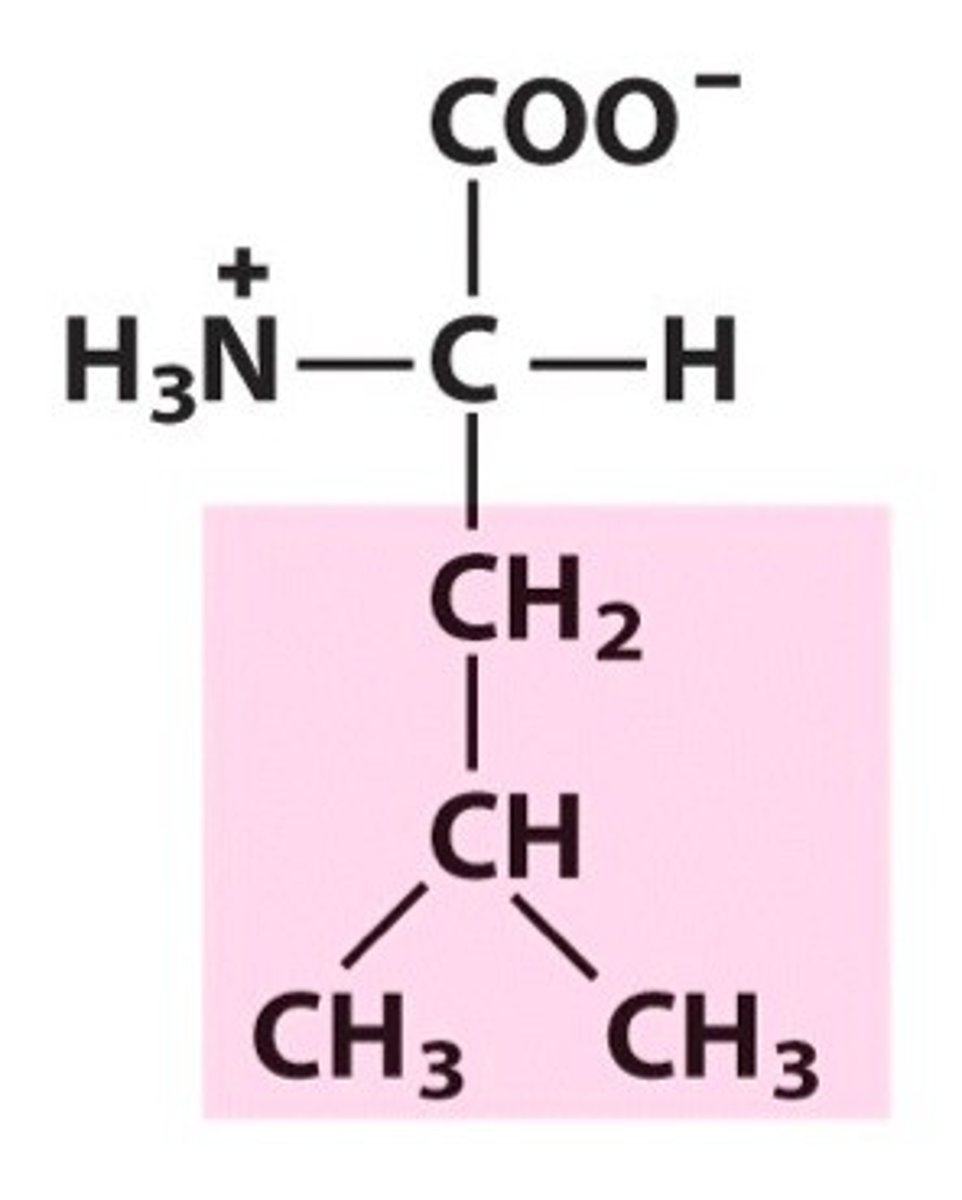
Structure of Isoleucine
Ile, I; isoleucine and leucine are structural isomers (lay-out of side chain differs). In leucine, side-chain forms an upside-down "Y" shape. In isoleucine, side-chain forms an upside down L shape (imagine the CH simplified as we saw in Leucine, and not with an extra dash).
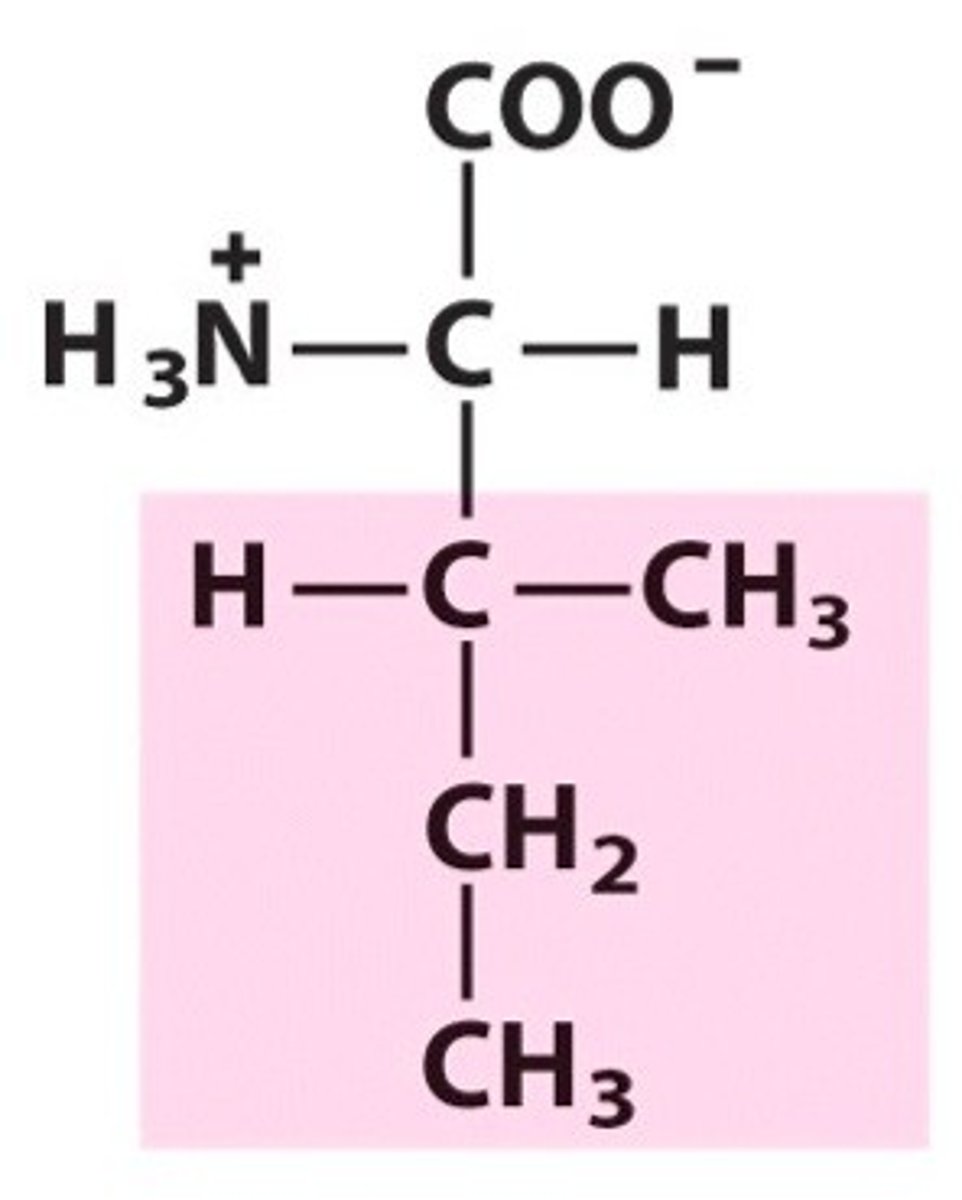
Structure of Methionine
Met, M; also known as a sulfur-containing amino acid.
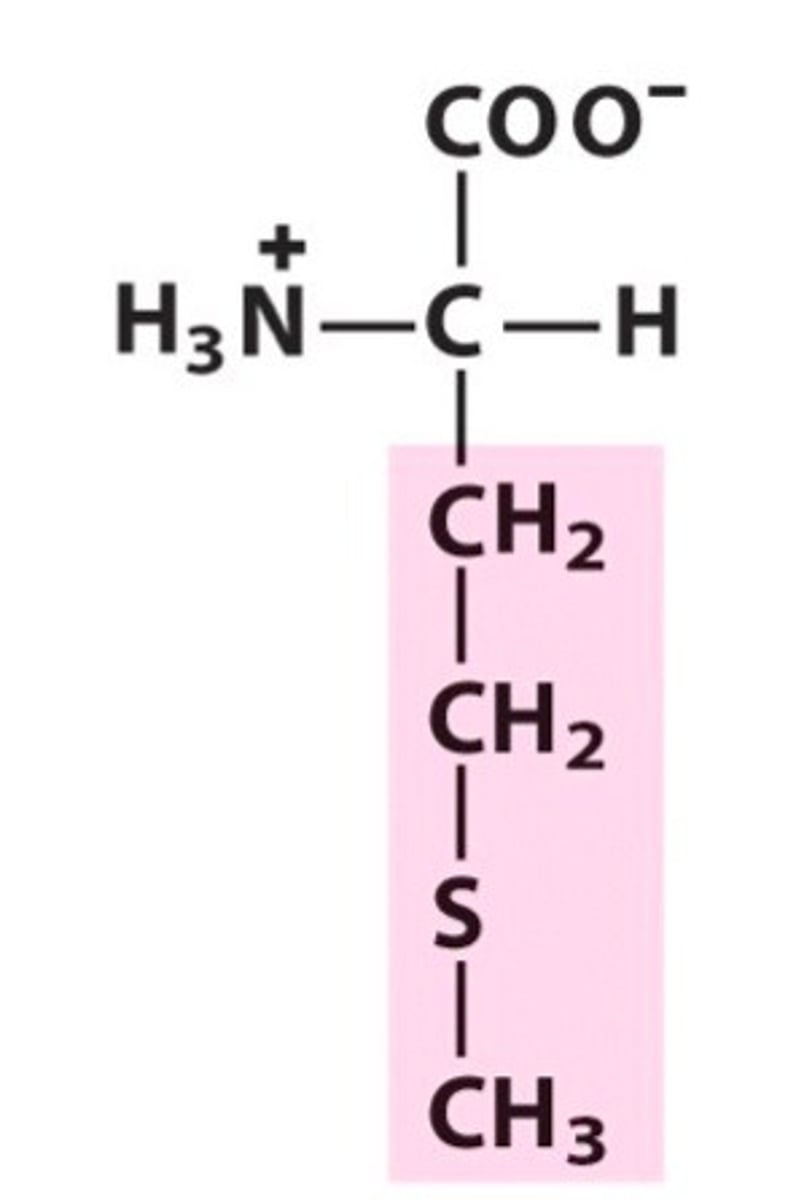
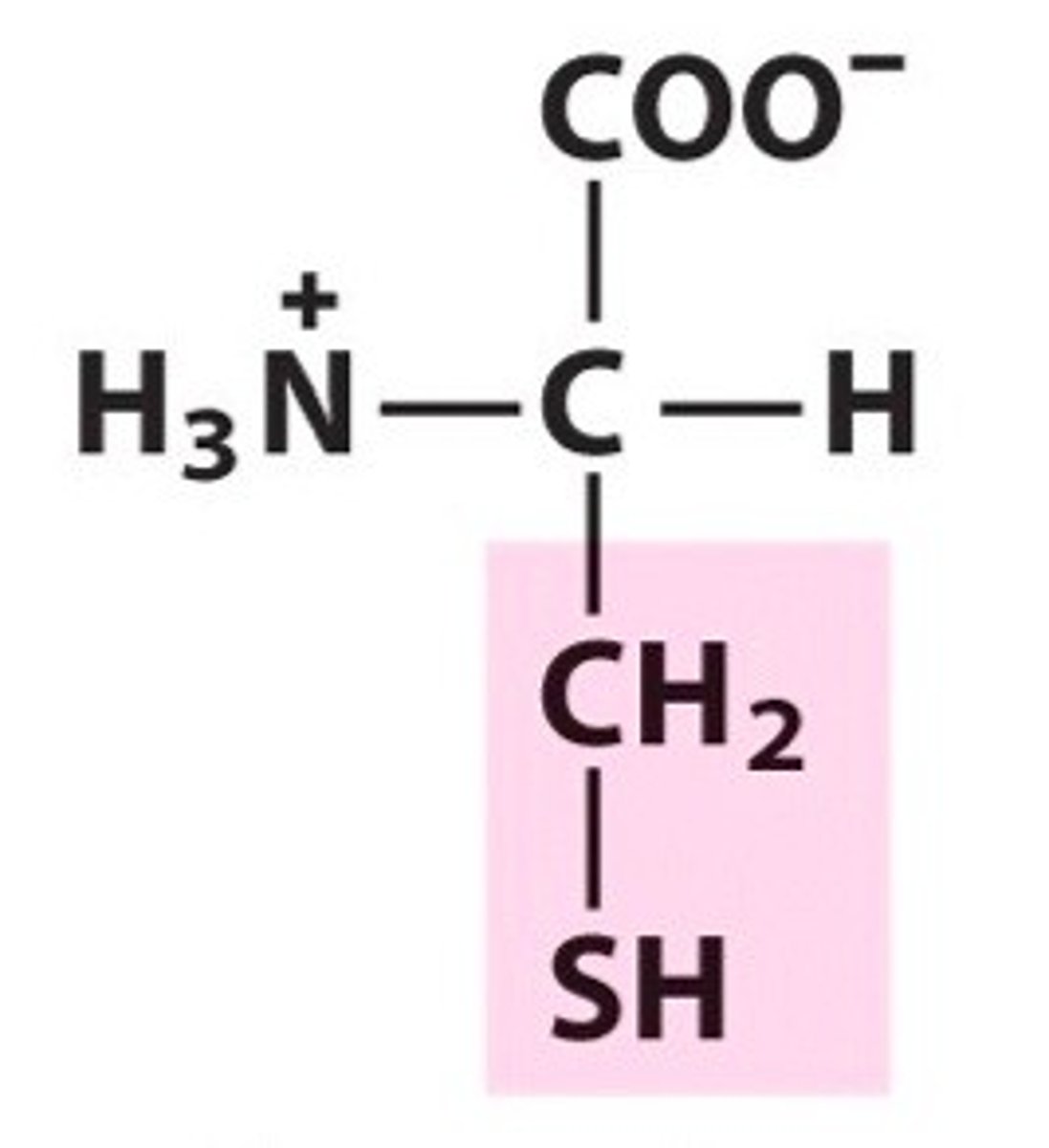
Structure of Cysteine
Cys, C; Take Met's structure, get rid of one methyl group and one CH2. Replace "S" with "SH" bond.
Sulfur-containing amino acids: METeor CYSts Contain Sulfur!
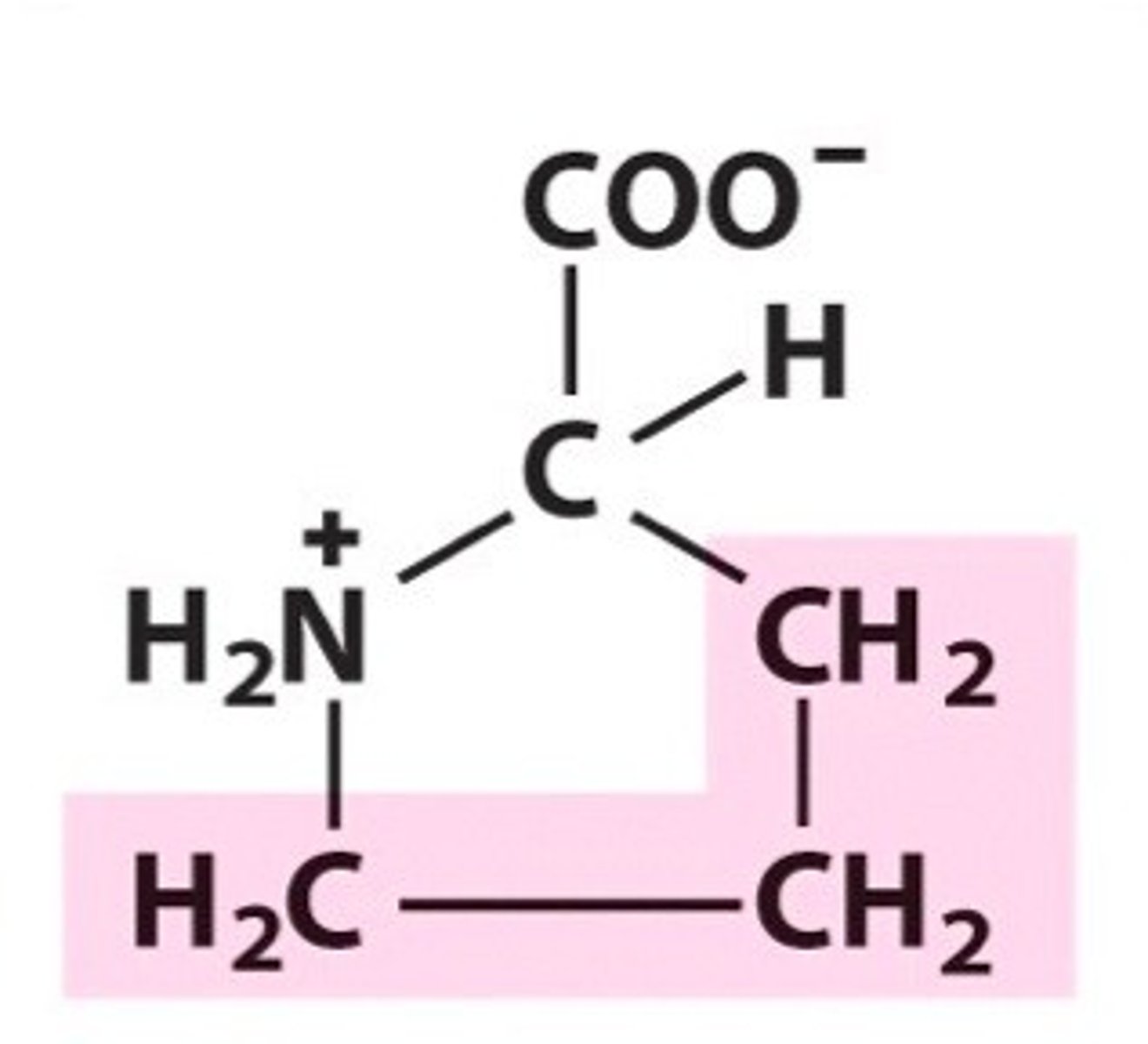
Structure of Proline
Pro, P; R group has a ring structure consisting of 3 CH2's, with one CH having taken an "H" from NH3+, which is now NH2+.
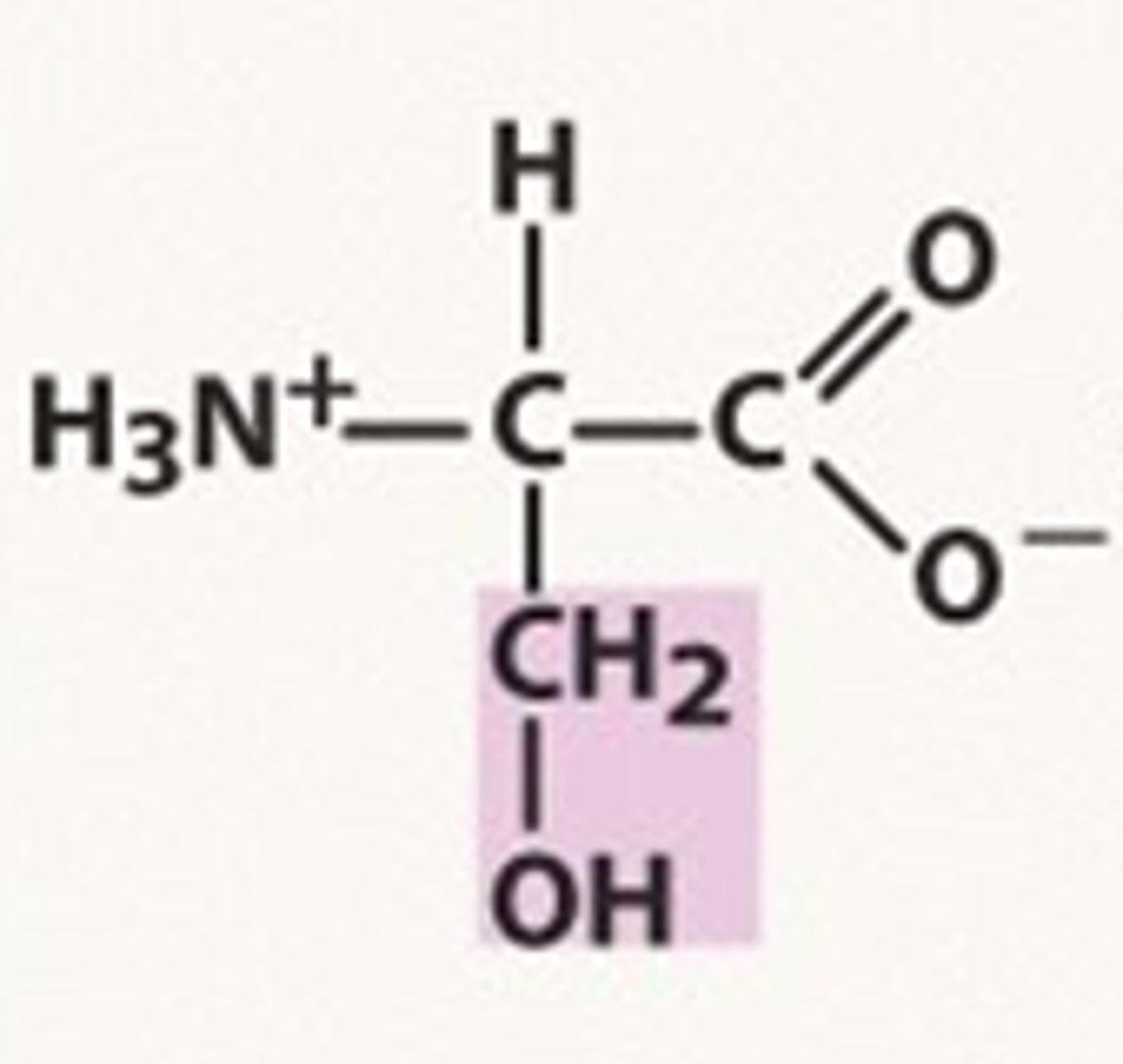
Structure of Serine
Ser, S; one of two alcohol amino acids. Similar structure as Cysteine -- both start off with CH2, and have a second component in the side-chain which ends in an H. For serine it's -oH; for cysteine, it's -sH.
Structure of Threonine
Thr, T; similar to serine's structure but has an extra methyl group attached -- can think of removing the "H" from CH2 to form the methyl group). One of two alcohol amino acids (so will contain a hydroxide).
Comparing Serine and Threonine: Serine has 2 syllables and thus 2 substituents in its R group, one of them being CHTWO. Threonine has 3 syllables, is spelled THRE(E)onine and thus has 3 substituents in its R group, one of them being CHTHREE -- or can think of as an isopropyl group where one methyl group is replaced by a hydroxyl group (HO-).
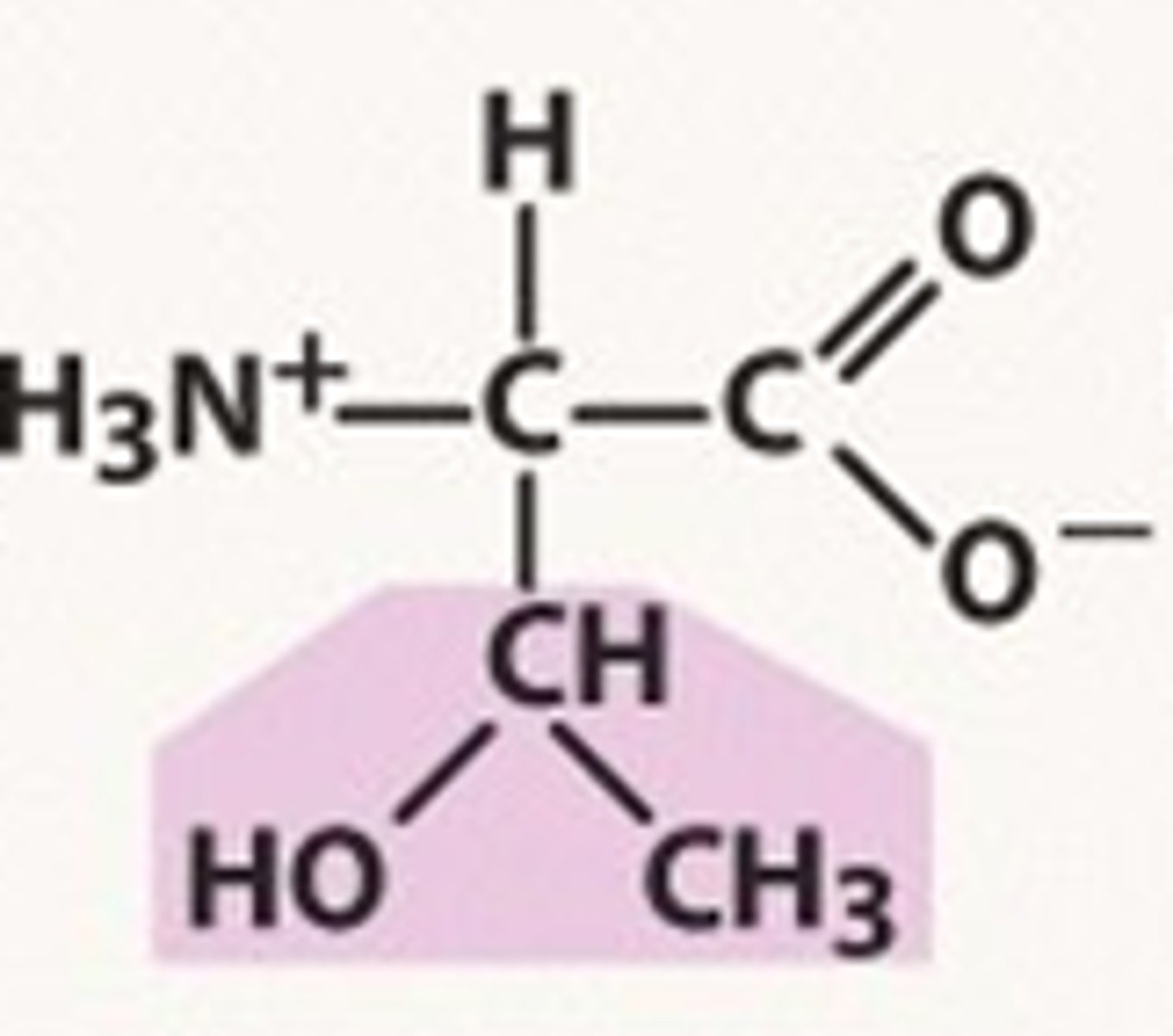
How can we remember the alcohol amino acids?
Alcohol is a SERious THREat. Serine comes first -- by memorizing serine's structure, we have an easier time learning threonine's structure!
Structure of Asparagine
Asn, N. Can think of the side-chain (R group) as having a central carbon with 3 different substituents: CH2, an amino group, and an oxygen (double bond present). One of two amide amino acids. As there are two A's present in "Asparagine", there's a double bond in the side-chain. It also has double -H2 groups: NH2, and CH2.
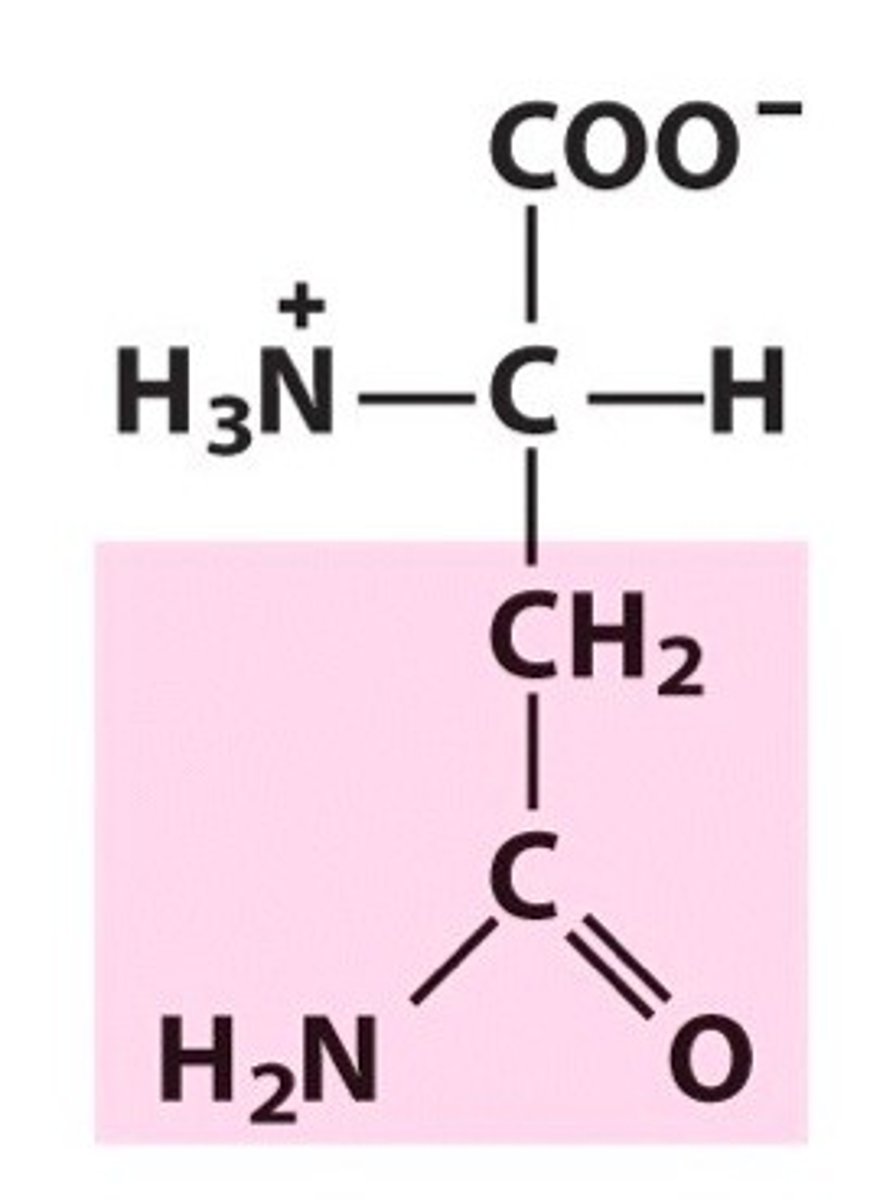
Structure of Glutamine
Gln, Q; just as gluttonous individuals get bigger, so will glutamine! Take Asn's structure and add a CH2. One of two amide amino acids.
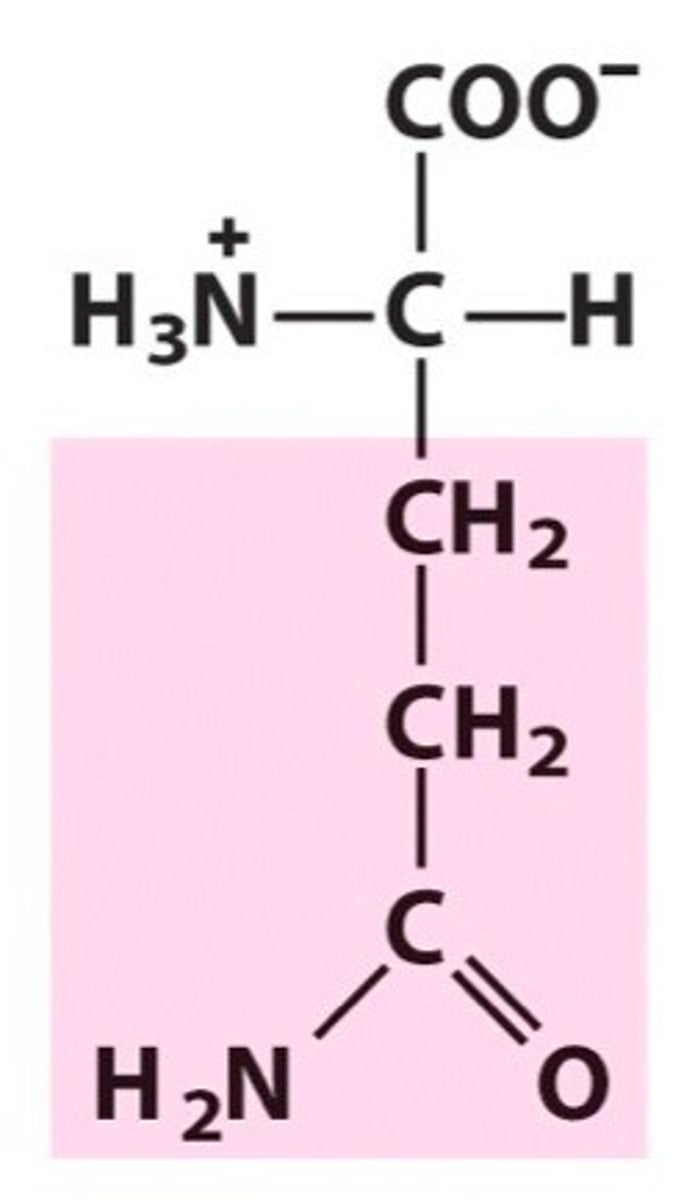
How can we remember the amide amino acids?
Amid this ASPARAGus is a GLUTiNous quail.
Structure of Histidine
His, H; memorizing His's structure makes it easier to understand the other's. Describing the ring: we have CH in the middle on both sides (going inside out: HC vs CH). They both connect via single bond to NH. C_N and C=CH is where the ring begins. N from C_N is double bonded to HC.
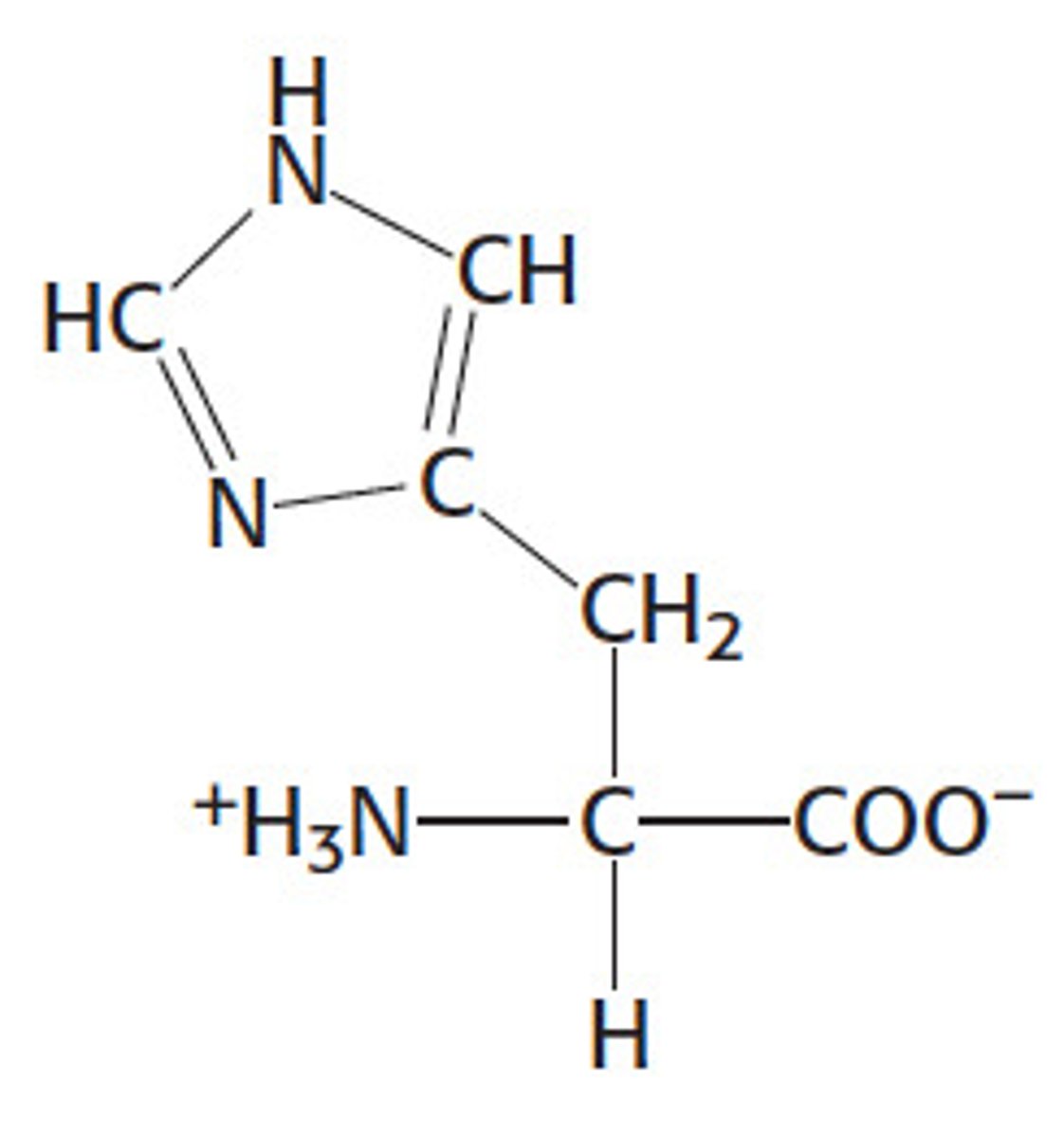
Structure of Lysine
Lys, K; take a Histidine, remove the two double bonds and the bond b/t the N with a lone pair and the carbon on its left. Next, remove the NH. Change the N with a lone pair to a NH3+. This is Lysine. To remember the 4 CH2's for Lysine, can think of "Lice". Lice contains FOUR letters, just as Lys contains FOUR CH2 groups.
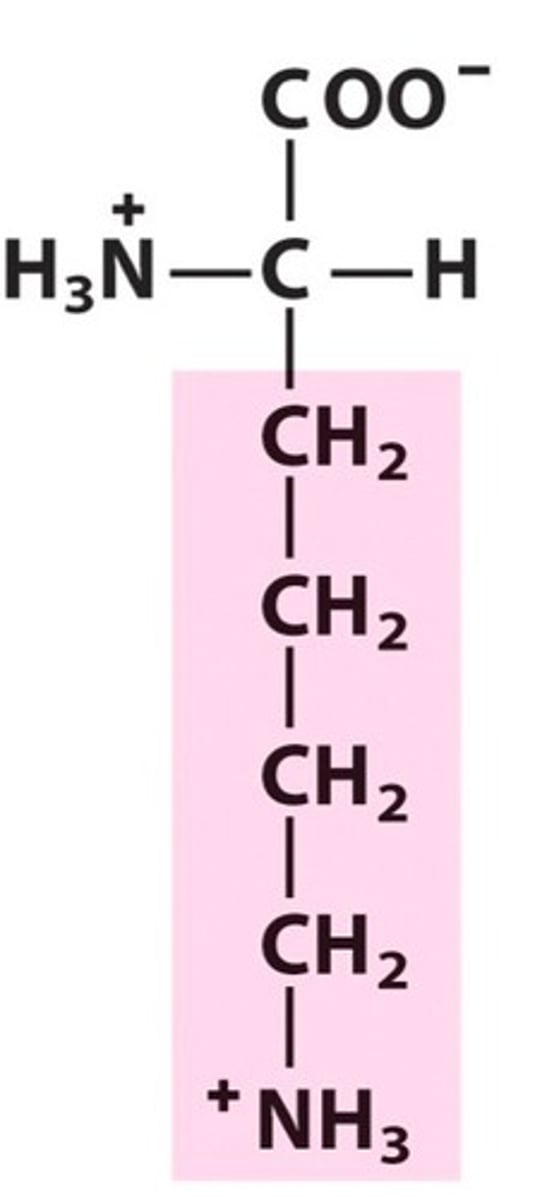
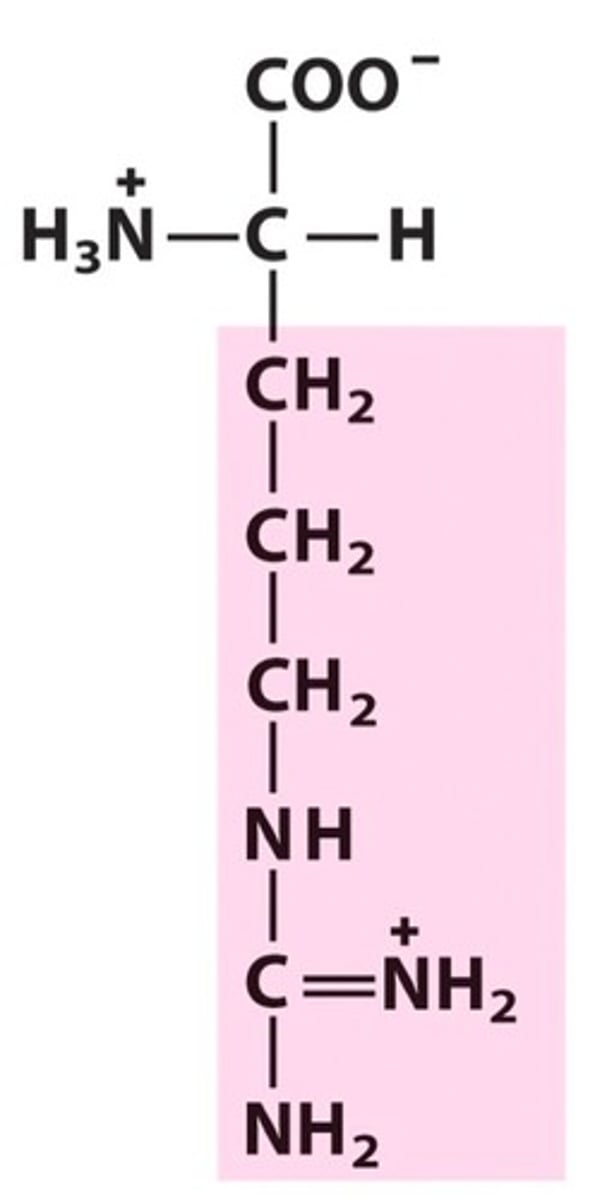
Structure of Arginine
Arg, R; to change lysine into arginine, switch NH3+ with the bottom-most CH2. Change NH3+ to NH, and CH2 to C. Draw in two NH2 next to the C, one of them having a double bond since Carbon makes 4 bonds. As a result, that nitrogen with a double bond will have a positive charge.
Not only is Lysine a smaller word than Arginine, so is its structure smaller than Arginine's. Thus we remember that this structure is Arginine's because it's longer.
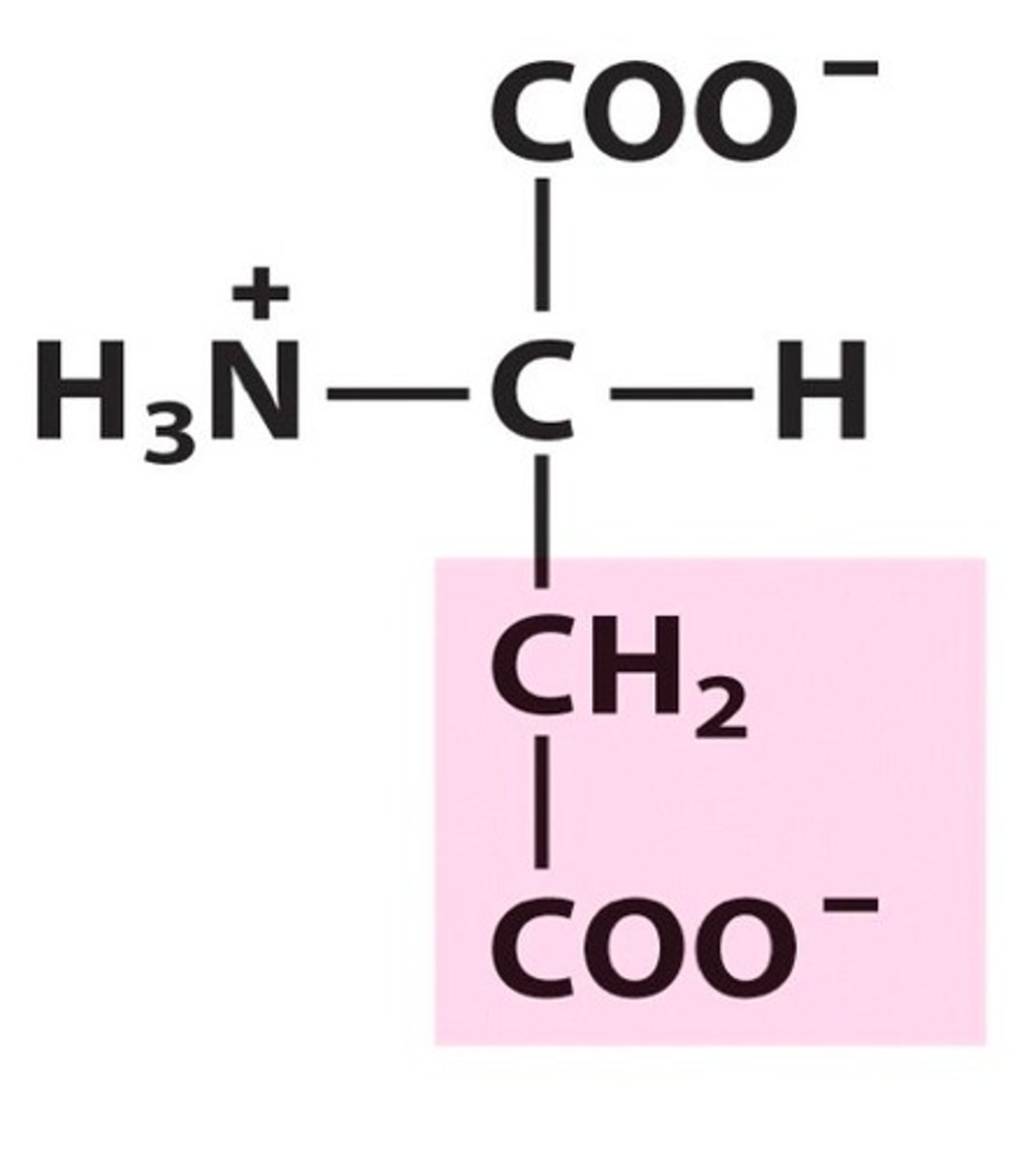
Structure of Aspartate
Asp, D
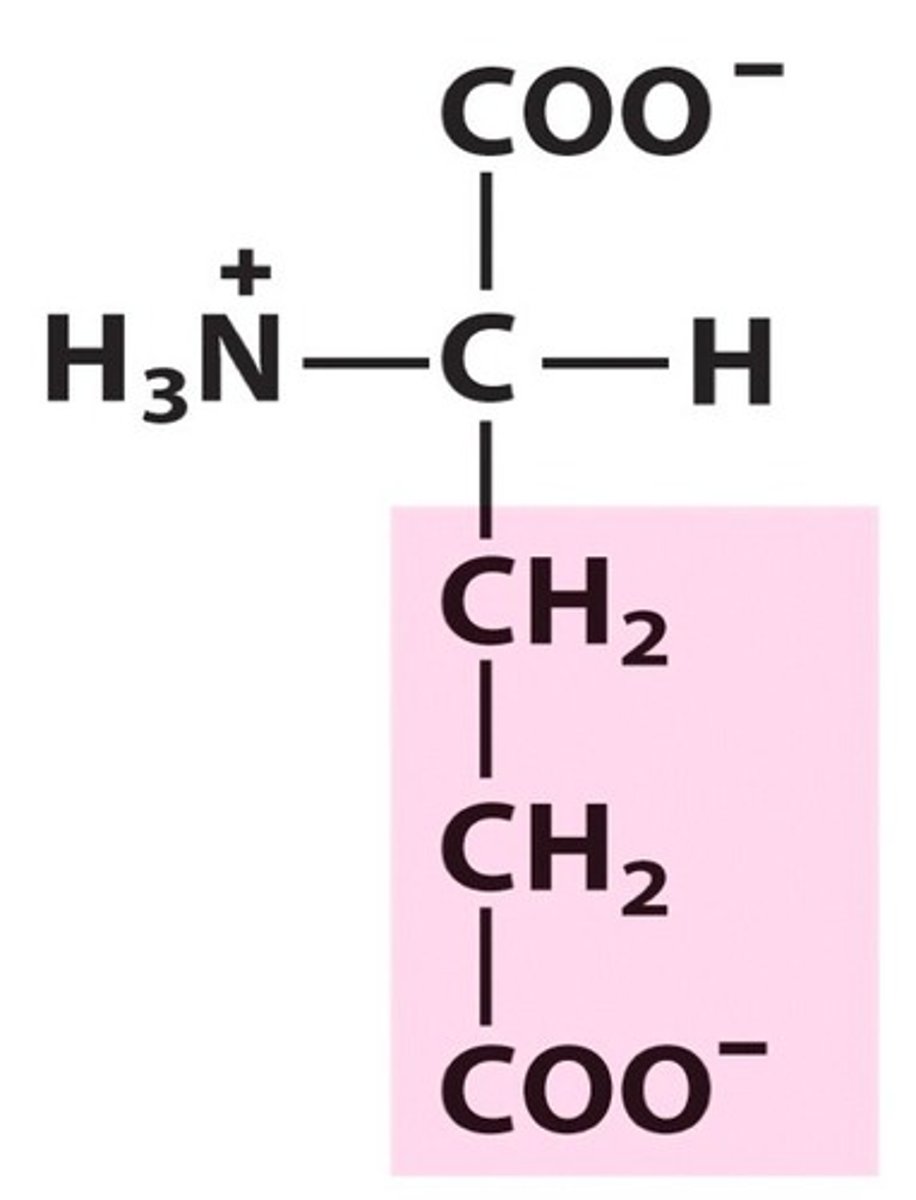
Structure of Glutamate
Glu, E; take aspartate and add another CH2 group. It's pretty gluttonous!
What does Aspartate/Glutamate have in common with Asparagine/Glutamine?
The amino acids starting with "Glu" have the same exact formula as the amino acids starting with "Asp", they just have an extra CH2 group! The amino acids starting with "Asp" have a CH2 group and some other substituent(s), the "Glu" acids just have an extra CH2.
Structure of Phenylalanine
Phe, F; Benzyl attached to it. 6 membered ring.
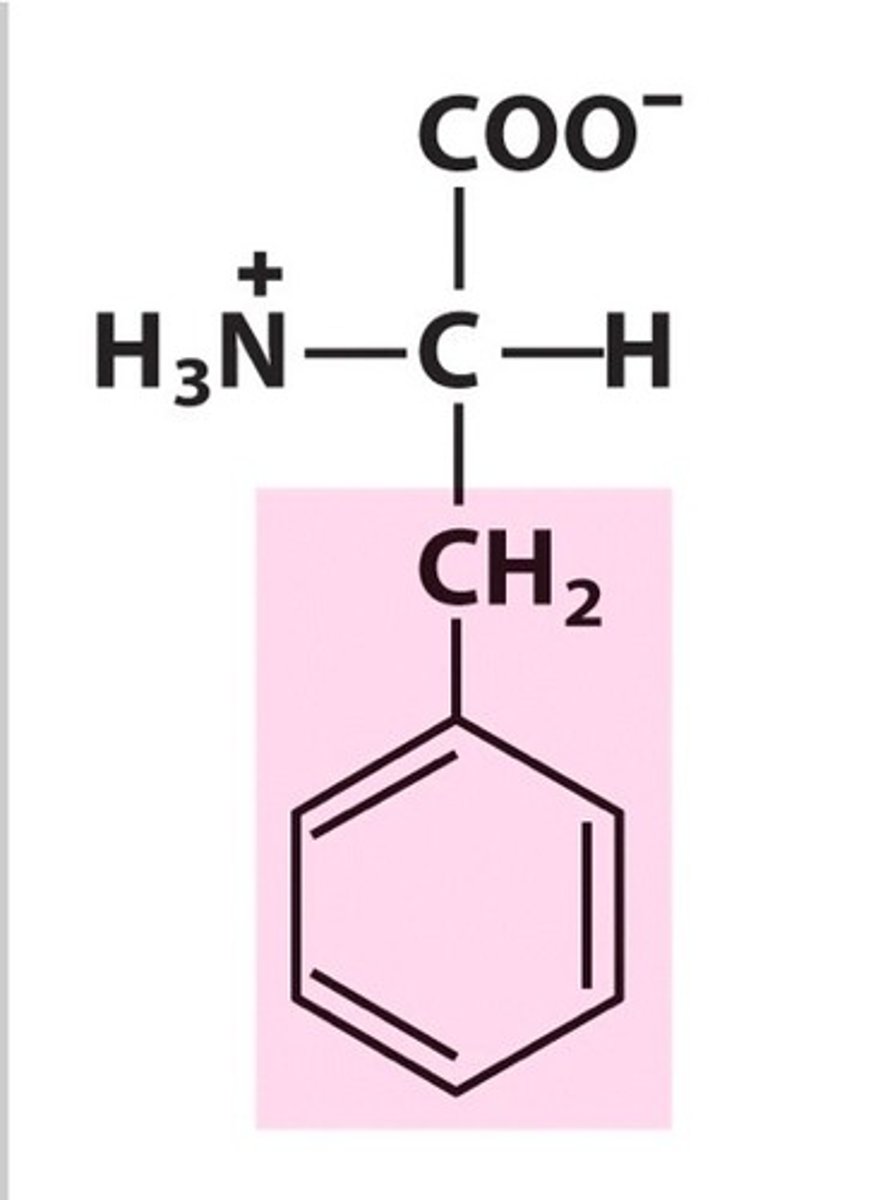
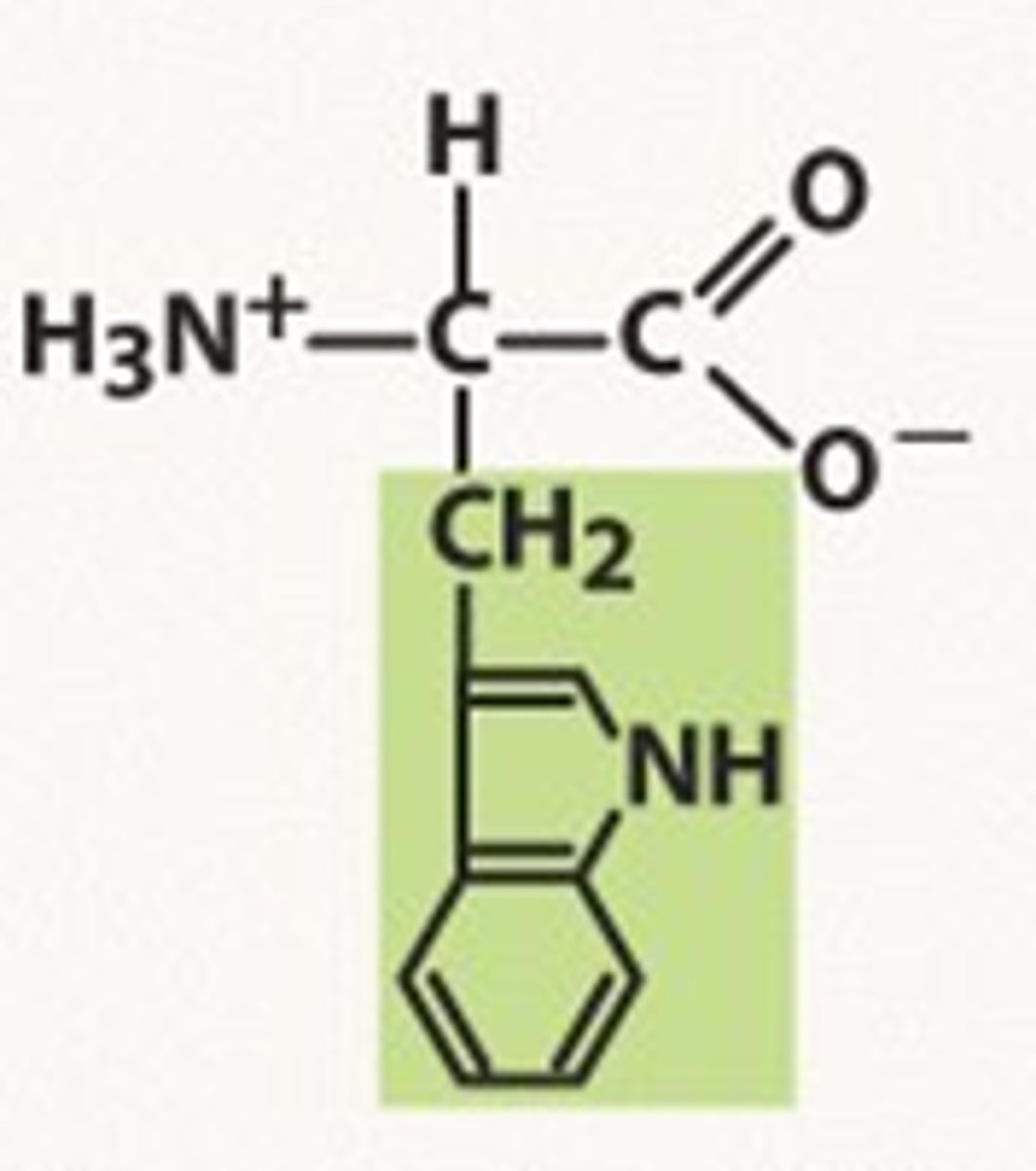
Structure of Tryptophan
Trp, W; has two aromatic rings: one a five-member ring with a nitrogen, and one a six-membered ring.
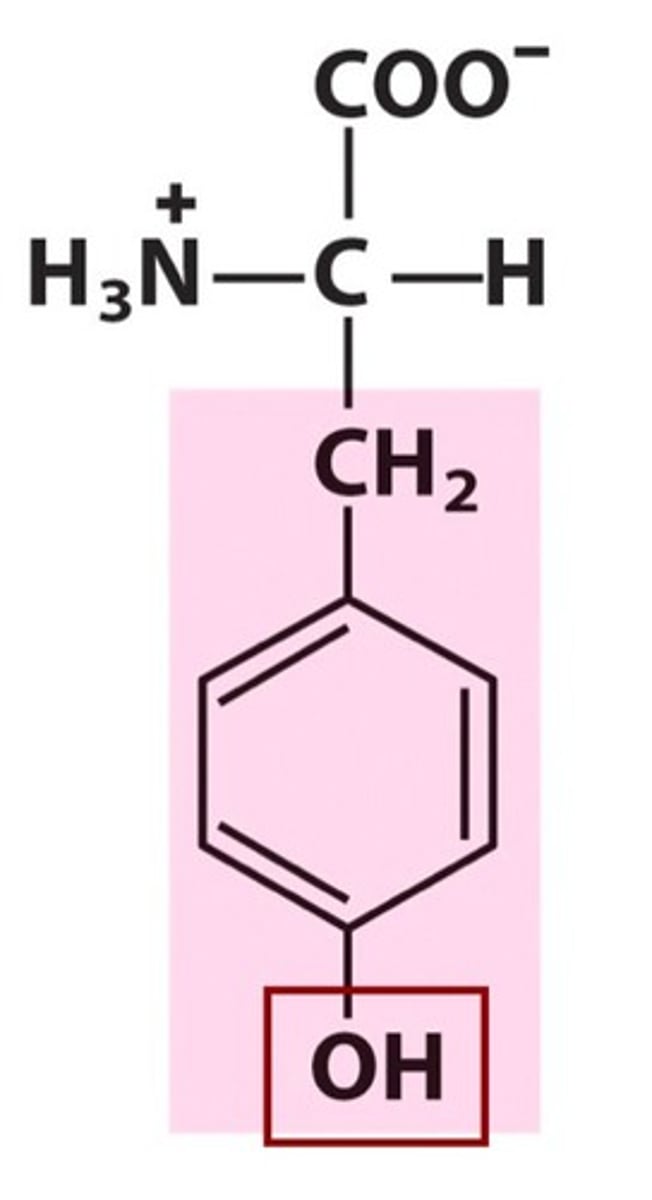
Structure of Tyrosine
Tyr, Y; almost identical to Phe but has a hydroxyl group.
All the aromatic amino acids have a CH2 out front and some sort of ring at the bottom of side-chain. Tryptophan has two rings, similar to how a "W" looks like two V's. Tip to help you remember structure: Just as in WFY, F (Phe) and Y (Tyr) are closer together, they are the most similar in structure.
Amino acids are also ___________________ species.
Amphoteric; amino acids are amphoteric compounds, as they contain both an acidic carboxylic acid group (COOH) and a basic amino group (NH2).
Amino acids, with their acidic carboxyl group and basic amino group, are amphoteric molecules. That is, they can act as both acids and bases. Amino groups can take on a positive charge by being protonated, and carboxyl groups can take on negative charges by being deprotonated.
How to distinguish between acidic and basic compounds?
COO- becomes neutral when it accepts a proton (COOH). NH3+ becomes neutral when it donates a proton. Or acidic compounds (like COOH) have lower pKas, and basic compounds have higher pKas.
Amphoteric species can either ______________ a proton or ________________ a proton.
accept (relative to its basic nature), donate (relative to its acidic nature); how they react depends on the pH of their environment. In basic solutions, the amino acid can become fully deprotonated; in acidic solutions, it can become fully protonated.
What two facts can we use to understand the behavior of amino acids?
1.) ionizable groups' behavior under acidic and basic conditions.
2.) what the pKa of a group specifies.
What is an ionizable group's behavior under acidic and basic conditions?
Ionizable groups tend to gain protons under acidic conditions and lose them under basic conditions. So, in general, at low pH, ionizable groups tend to be protonated; at high pH, they tend to be deprotonated..
What are an amino acid's ionizable groups?
The functional groups in amino acids in the enzyme have the ability to readily ionize. Each amino acid has at least two acid-base groups (an amino group and a carboxyl group). Certain amino acids within enzyme may also have ionizable side chains. Seven of the amino acids- arginine, aspartic acid, cysteine, glutamic acid, histidine, lysine and tyrosine; have ionizable side chains. These are able to donate or accept protons due to their ionizable side chains.
At physiological pH (pH = ~7.4) the -NH2 group remains basic and protonated while the -COOH group is acidic and remains deprotonated.
What is deprotonation?
The removal of a proton (hydrogen without electrons) from an acid by a base. Acids undergo deprotonation.
Which seven amino acids have ionizable side-chains?
arginine, aspartic acid, cysteine, glutamic acid, histidine, lysine and tyrosine have ionizable side chains. These side-chains are able to donate or accept protons. Way to remember the seven amino acids: DECKRYH - Dragons Eat Crazy-Knights Riding Yellow Horses.
What does the pKa of a group specifies?
The pKa of a group is the pH at which, on average, half of the molecules of that species are deprotonated; that is, [protonated version of the ionizable group] = [deprotonated version of the ionizable group] or [HA] = [A - ]. Equal concentrations of protonated version and deprotonated version in the solution.
What happens when the pH is less than the pKa or more than the pKa?
If the pH is less than the pKa , a majority of the species will be protonated. If the pH is higher than the pKa , a majority of the species will be deprotonated.
How many pKa values do amino acids have at least?
Two. Because all amino acids have at least two groups that can be deprotonated (their two ionizable groups: carboxyl group and amino group), they all have at least two pKa values.
What is pKa (1), and what is its usual numerical value?
(the first pKa value) the pKa for the carboxyl group and is usually around 2.
What is pKa (2), and what is its usual numerical value?
(the second pKa value) the pKa for the amino group, which is usually between 9 and 10.
For amino acids with ionizable side chains, how many pKa values will they have?
For amino acids with an ionizable side chain, there will be THREE pKa values. (p.88)
Glycine + pKa example!
At pH 1, because we're far below the pKa of the amino group, the amino group will be fully protonated (-NH3+)and thus positively charged.
Because we're also below the pKa of the carboxylic acid group, it too will be fully protonated (-COOH) and thus neutral. Therefore, at very acidic pH values, amino acids tend to be positively charged
If we increase the pH of the amino acid solution from pH 1 to pH 7.4, the normal pH of human blood, we've moved far above the pKa of the carboxylic acid group. Thus, at physiological pH, you will not find amino acids with the carboxylate group protonated (-COOH). Under these conditions, the carboxyl group will be in its conjugate base form and be deprotonated, becoming (-COO^-) .
Conversely, we're still well below the pKa of the basic amino group, so it will remain fully protonated and in its conjugate acid form (-NH3+ and not -NH2).
Thus, we have a molecule that has both a positive charge and a negative charge, but overall, the molecule is electrically neutral. We call such molecules dipolar ions, or zwitterions.
The two charges neutralize one another, and zwitterions exist in water as internal salts.
When is the amino acid positively charged?
Under acidic conditions, like at a pH of 1, the carboxyl group is neutral (protonated), and the amino group is positively charged (protonated), thus the amino acid is positively charged. Therefore, at very acidic pH values, amino acids tend to be positively charged (b/c neither functional groups are deprotonated).
Amino acid in an acidic solution vs in a more basic solution!
Amino acid becomes a zwitterion in a pH far greater than 2. The amino acid is neutral.
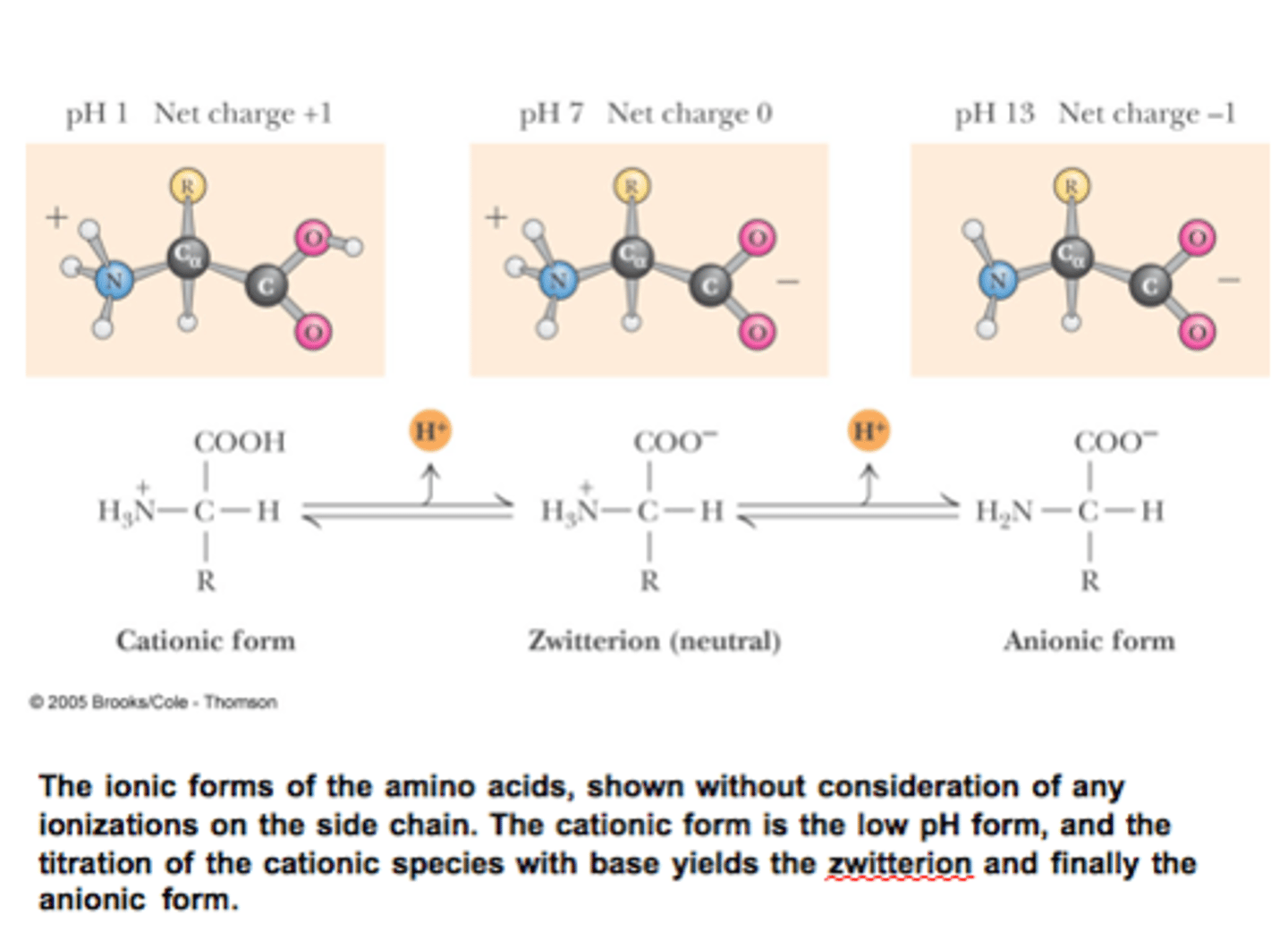
When is the amino acid neutral in charge?
At an intermediate pH (for example, pH of 7.4) where only the carboxyl group is deprotonated and the amino group remains protonated.
When is the amino acid (glycine) negative in charge?
At more basic pH -- for example, those above 9 or 10! The amino group will be deprotonated and have a neutral charge. Since the deprotonated carboxyl group is negatively charged, the amino acid will have an overall negative charge. Milk of magnesia, which is often used as an antacid, has a pH around 10.5. At that pH, the carboxylate group is already deprotonated and thus remains -COO^- . On the other hand, we are now well above the pKa for the amino group, so it deprotonates too, becoming -NH2 .
So, at highly basic pH, glycine is now negatively charged.
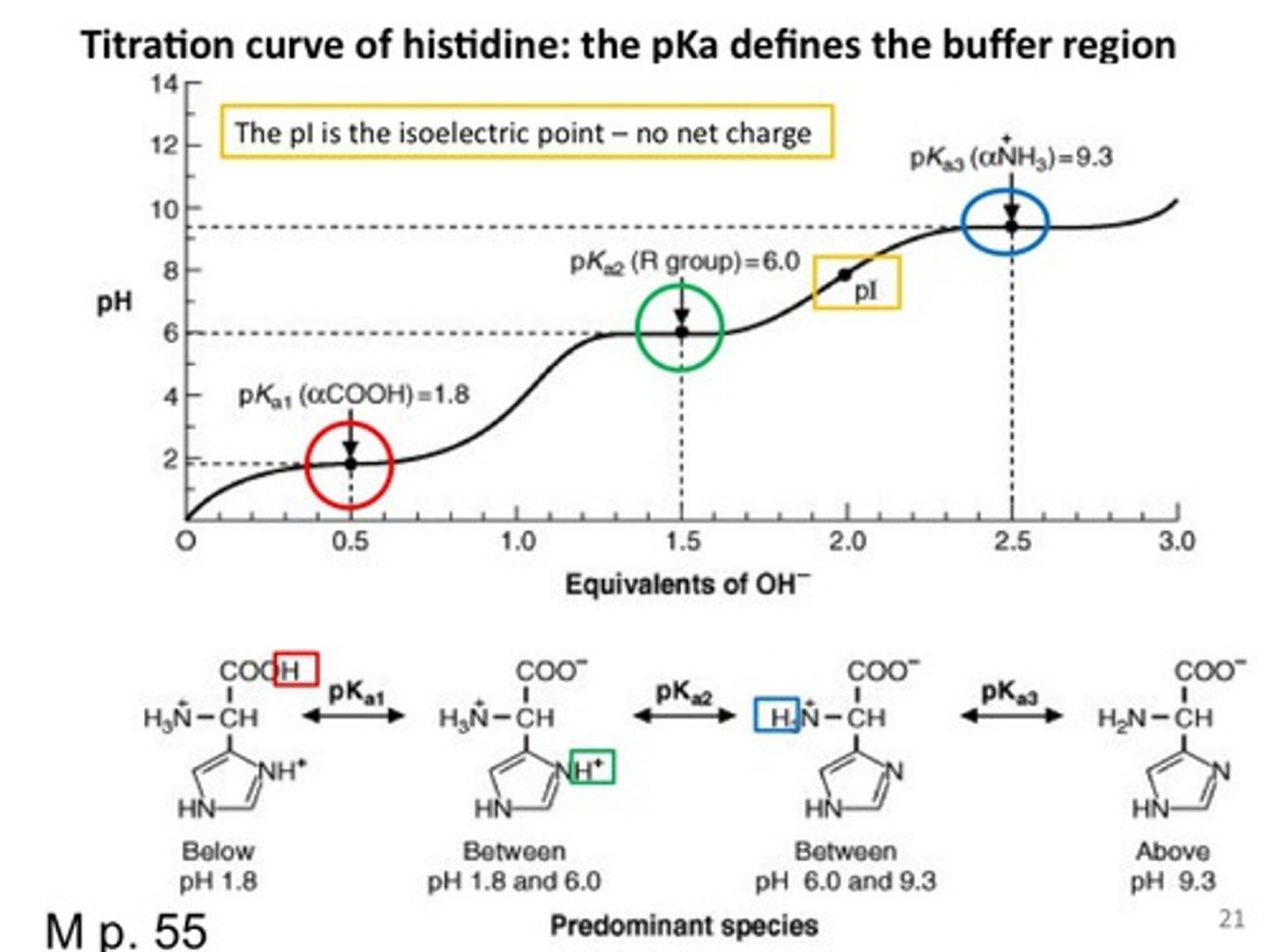
Understanding the Titration Curve for Glycine
Imagine an acidic 1 M (the concentration) glycine solution. At low pH values, glycine exists predominantly as +NH3CH2COOH; it is fully protonated (meaning both carboxyl and amino groups are protonated), with a positive charge. NaOH is a strong base and strong bases are capable of deprotonating weak acids, like carboxylic acids (R-COOH) and NH3+! NH3+ is usually an base but can act as an acid under certain pHs. As the solution is titrated with NaOH (and solution becomes more and more basic), the carboxyl group will deprotonate first because it is more acidic than the amino group.
When 0.5 equivalents of base have been added to the solution, the concentrations of the fully protonated glycine and its zwitterion, +NH3CH2COO- , are equal; that is, [+NH3CH2COOH] = [+NH3CH2COO- ]. At this point, the pH equals pKa (1).
Remember: when the pH is close to the pKa, value of a solute, the solution is acting as a buffer and the titration curve is relatively flat.
As we add more base, the carboxylate group goes from half-deprotonated to fully deprotonated (So we don't have any more fully protonated glycines in the solution anymore since all carboxyl groups are deprotonated). The amino acid stops acting like a buffer, and pH starts to increase rapidly during this phase. When we've added 1.0 equivalent of base, glycine exists exclusively as the zwitterion form (remember, we started with 1.0 equivalent of glycine).This means that every molecule is now electrically neutral, and thus the pH equals the isoelectric point (pI) of glycine. This is true of all amino acids: the isoelectric point is the pH at which the molecule is electrically neutral.
As we continue adding base, glycine passes through a second buffering phase as the amino group deprotonates; again, the pH remains relatively constant. When 1.5 equivalents of base have been added, the concentration of the zwitterion form equals the concentration of the fully deprotonated form; that is, [+NH3CH2COO- ] = [NH2CH2COO - ], and the pH equals pKa2. Once again, the titration curve is nearly horizontal. Finally, when we've added 2.0 equivalents of base, the amino acid has become fully deprotonated, and all that remains is NH2CH2COO - ; additional base will only increase the pH further.
![<p>Imagine an acidic 1 M (the concentration) glycine solution. At low pH values, glycine exists predominantly as +NH3CH2COOH; it is fully protonated (meaning both carboxyl and amino groups are protonated), with a positive charge. NaOH is a strong base and strong bases are capable of deprotonating weak acids, like carboxylic acids (R-COOH) and NH3+! NH3+ is usually an base but can act as an acid under certain pHs. As the solution is titrated with NaOH (and solution becomes more and more basic), the carboxyl group will deprotonate first because it is more acidic than the amino group.</p><p>When 0.5 equivalents of base have been added to the solution, the concentrations of the fully protonated glycine and its zwitterion, +NH3CH2COO- , are equal; that is, [+NH3CH2COOH] = [+NH3CH2COO- ]. At this point, the pH equals pKa (1).</p><p>Remember: when the pH is close to the pKa, value of a solute, the solution is acting as a buffer and the titration curve is relatively flat.</p><p>As we add more base, the carboxylate group goes from half-deprotonated to fully deprotonated (So we don't have any more fully protonated glycines in the solution anymore since all carboxyl groups are deprotonated). The amino acid stops acting like a buffer, and pH starts to increase rapidly during this phase. When we've added 1.0 equivalent of base, glycine exists exclusively as the zwitterion form (remember, we started with 1.0 equivalent of glycine).This means that every molecule is now electrically neutral, and thus the pH equals the isoelectric point (pI) of glycine. This is true of all amino acids: the isoelectric point is the pH at which the molecule is electrically neutral.</p><p>As we continue adding base, glycine passes through a second buffering phase as the amino group deprotonates; again, the pH remains relatively constant. When 1.5 equivalents of base have been added, the concentration of the zwitterion form equals the concentration of the fully deprotonated form; that is, [+NH3CH2COO- ] = [NH2CH2COO - ], and the pH equals pKa2. Once again, the titration curve is nearly horizontal. Finally, when we've added 2.0 equivalents of base, the amino acid has become fully deprotonated, and all that remains is NH2CH2COO - ; additional base will only increase the pH further.</p>](https://knowt-user-attachments.s3.amazonaws.com/ad157712-8291-478a-9eeb-047972055e97.png)
What is the isoelectric point?
The isoelectric point is the pH at which the molecule is electrically neutral. This is true of all amino acids.
How can we calculate the isoelectric point for neutral amino acids (amino acids with no charged side-chains)?
It can be calculated by averaging the two pKa values for the amino and carboxyl groups: for glycine, the pI value is (2.34 + 9.60) ÷ 2 = 5.97.
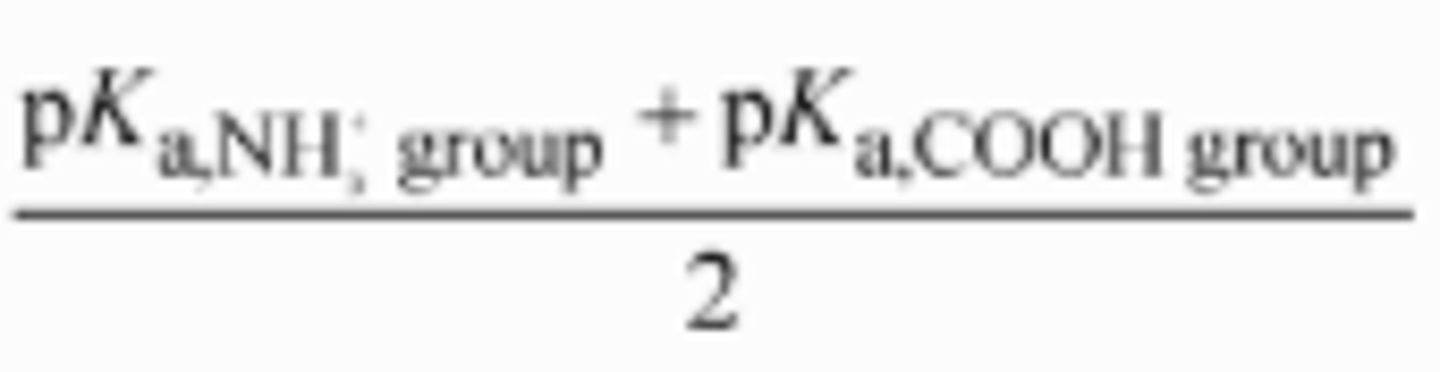
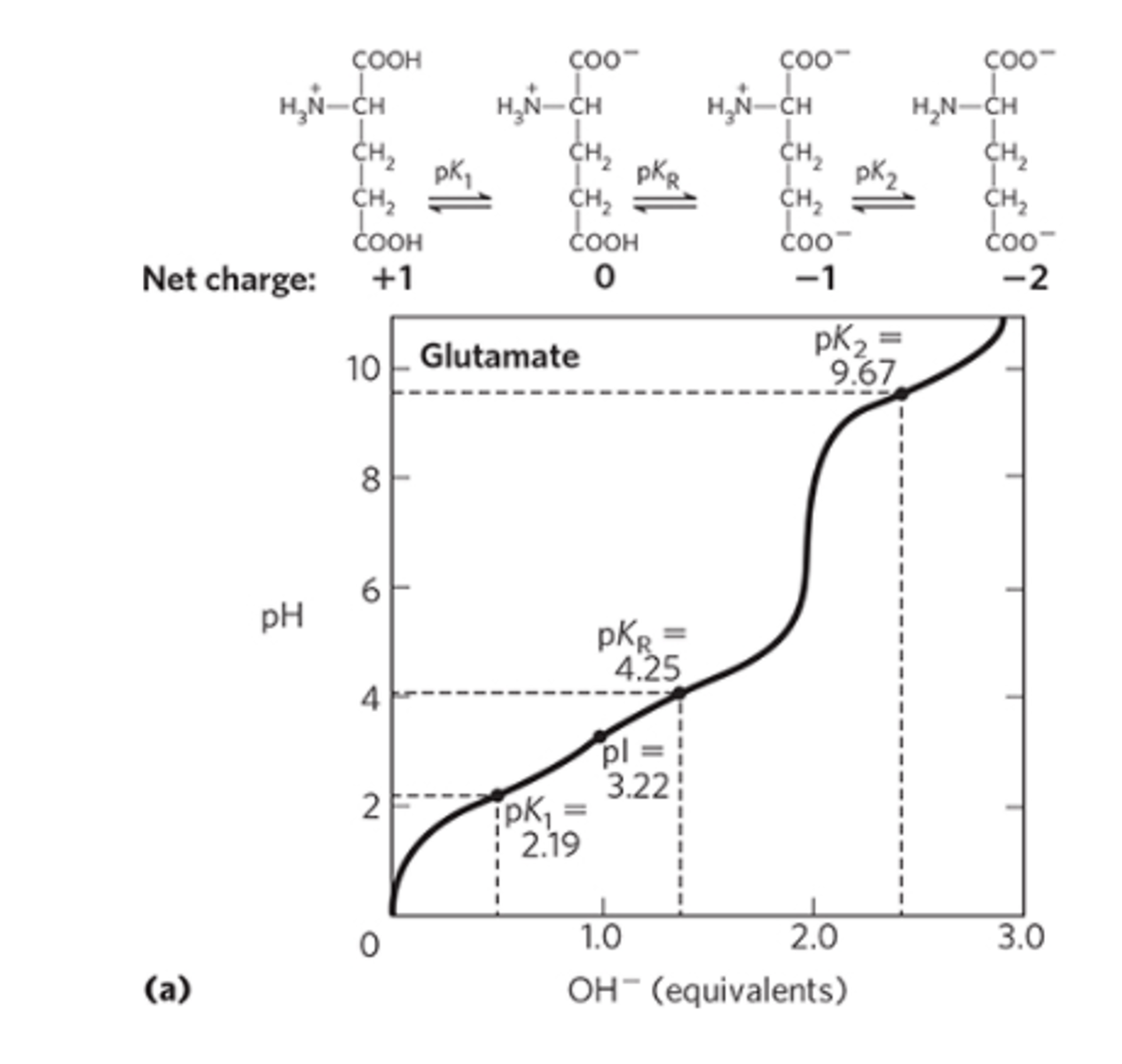
How does the titration curve look like for amino acids with an ionizable side-chain? (Glutamic Acid)
For amino acids with charged side chains, such as glutamic acid and lysine, the titration curve has an extra "step," but works along the same principles as described above. Let's envision glutamic acid!
Because glutamic acid has two carboxyl groups and one amino group, its charge in its fully protonated state is still +1. It undergoes the first deprotonation, losing the proton from its main carboxyl group, just as glycine does. At that point, it is electrically neutral. . When it loses its second proton, just as with glycine, its overall charge will be -1. However, the second proton that is removed in this case comes from the side-chain carboxyl group, not the amino group! The carboxyl group in the side-chain is relatively acidic and has a pKa of around 4.2.
The isoelectric point for an ACIDIC amino acid (or amino acid with negative side-chain) can be calculated as indicated in the picture. For glutamic acid, it's: (4.2+2.34)/2=3.27
When the amino group deprotonates, the charge of amino acid will remain negative.
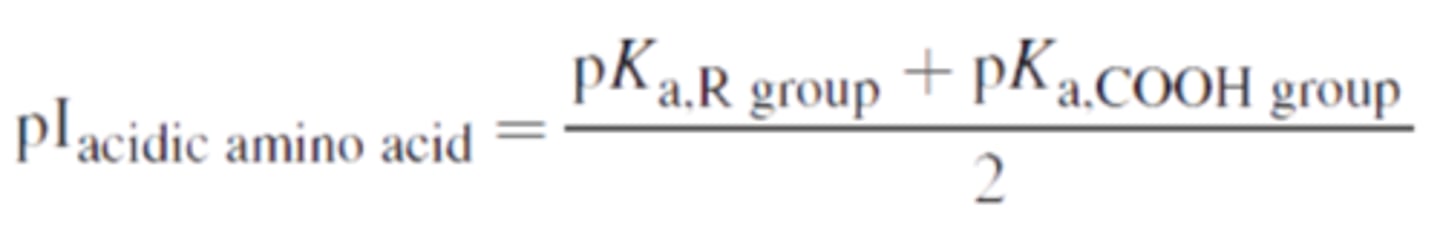
Amino acids with a negatively charged side-chain are ________________ (acidic/basic).
Acidic; they are DE -- aspartic acid (D), glutamic acid (E).
Amino acids with a positively charged side-chain are ______________ (acidic/basic).
Basic; they are KRH -- lysine (K), arginine (R), histidine (H).
How does the titration curve look like for amino acids with an ionizable side-chain? (Lysine)
Lysine, on the other hand, has two amino groups and one carboxyl group. Thus, its charge in its fully protonated state is +2, not +1. Losing the carboxyl proton, which still happens around pH 2, brings the charge down to +1. Lysine does not become electrically neutral until it loses the proton from its main amino group, which happens around pH 9. It gets a negative charge when it loses the proton on the amino group in its side chain, which happens around pH 10.5. Thus, the isoelectric point of lysine is the average of the pKa values for the amino group and side chain; the pI is around 9.75. The isoelectric point for a basic amino acid can be calculated as indicated by the image -- (9 + 10.5)/2 = 9.75.
pI point is a value between the pH when the amino acids start to get deprotonated to become zwitterions and when the pH when the amino acids start to become fully deprotonated (and AA gets neg. charge).
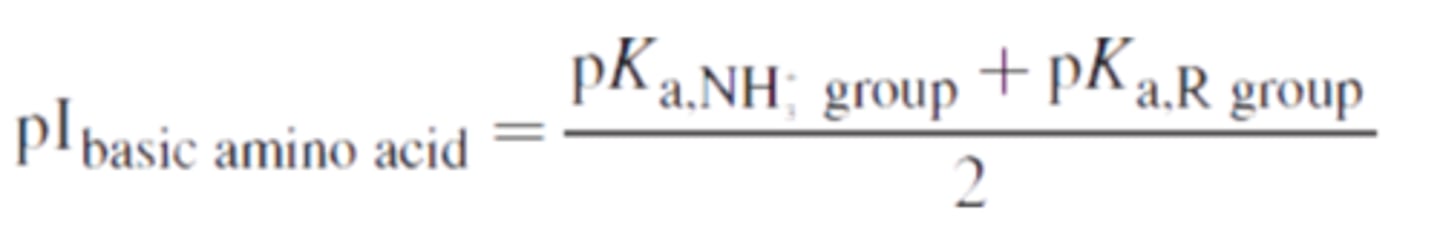
Titration Curve of Lysine
When we add .5 equivalents of base, half of the concentration now contains lysine molecules in which the carboxylate group is deprotonated (-COO^-), and the other half contains lysine molecules in which the main carboxylate group is fully protonated (-COOH). At this point, the pH is the pKa(1), and since the pH is near the pKa(1) value, the solution will act as a buffer (and the titration curve will straighten out). When we add 1.0 equivalent of base, ALL lysine molecules' carboxylate group is deprotonated. The charge of a lysine AA when its carboxylate group is deprotonated is +1, because w/o any base added (w/ 2 positively charged NH3+ groups, and one neutral COOH group), the AA had a charge of +2. So at 1.0 equivalent of base added, all lysine AA have a charge of +1. When we add 1.5 equivalents of base, half of the amino acids are electrically neutral since in half of the amino acids the main amino group undergoes deprotonation. Here, the pH is the pKa (2). When we add 2.0 equivalent of base, all amino acids in concentration have a main amino group that underwent deprotonation, and all lysine AA have a charge of 0. At this point is the isoelectric point since all molecules are electrically neutral.
Later on, when the amino group in the side chain is deprotonated, the AA will have a charge of -1.
Titration Curve of Glutamic Acid
When we add .5 equivalents of base, half of the concentration now contains glutamic acid molecules in which the main carboxylate group is deprotonated (-COO^-), and the other half contains glutamic acid molecules in which the main carboxylate group is fully protonated (-COOH). At this point, the pH is the pKa(1), and since the pH is near the pKa(1) value, the solution will act as a buffer (and the titration curve will straighten out). When we add 1.0 equivalent of base, ALL glutamic acid molecules' carboxylate group is deprotonated. Thus, since we started out at a +1 charge for the AA before adding any base (2 neutral -COOH's, one positively charged NH3+ group), when the main carboxylate acid group now has a negative charge, the glutamic acid AA now has a neutral charge. So when all the AA in solution have their carboxylate acid group deprotonated, all molecules are electrically neutral. At this point, the pH is the isoelectric (pI) point!
When we add more base, eventually the carboxylate acid group in the side chain will be deprotonated. At a pKa (2) of 4ish, we see that half of the glutamic acid molecules contain a deprotonated side-chain carboxylate acid group. At this point, AA with this deprotonated side chain have an eelctric charge of -1. When the amino acid group is deprotonated later on, charge of AA will remain -1.
Amino acids with acidic side chains have pI values ________(below/above) 6; amino acids with basic side chains have pI values ________ (below/above) 6.
below; above. Amino acids with acidic side chains have relatively low isoelectric points, while those with basic side chains have relatively high ones.
What are peptides composed of?
Peptides are composed of amino acid subunits, sometimes called residues.
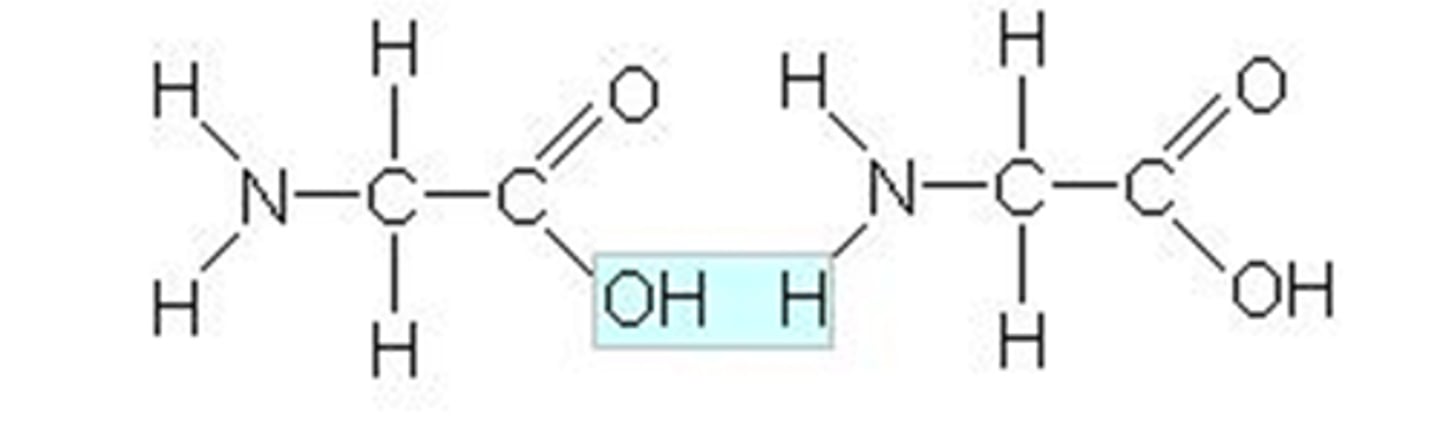
What distinguishes dipeptides from tripeptides?
Dipeptides consist of two amino acid residues; tripeptides have three.
When it comes to length of residue chains, what distinguishes oligopeptide from polypeptide?
The term oligopeptide is used for relatively small peptides, up to about 20 residues; while longer chains are called polypeptides
What bond joins together residues in peptides?
A peptide bond, which is a specialized form of an amide bond.
What is the structure of a peptide bond?
Peptide bond is a formation between the -COO- group of one amino acid/residue and the NH3+ group of another amino acid. This forms the functional group -C(O)NH-.
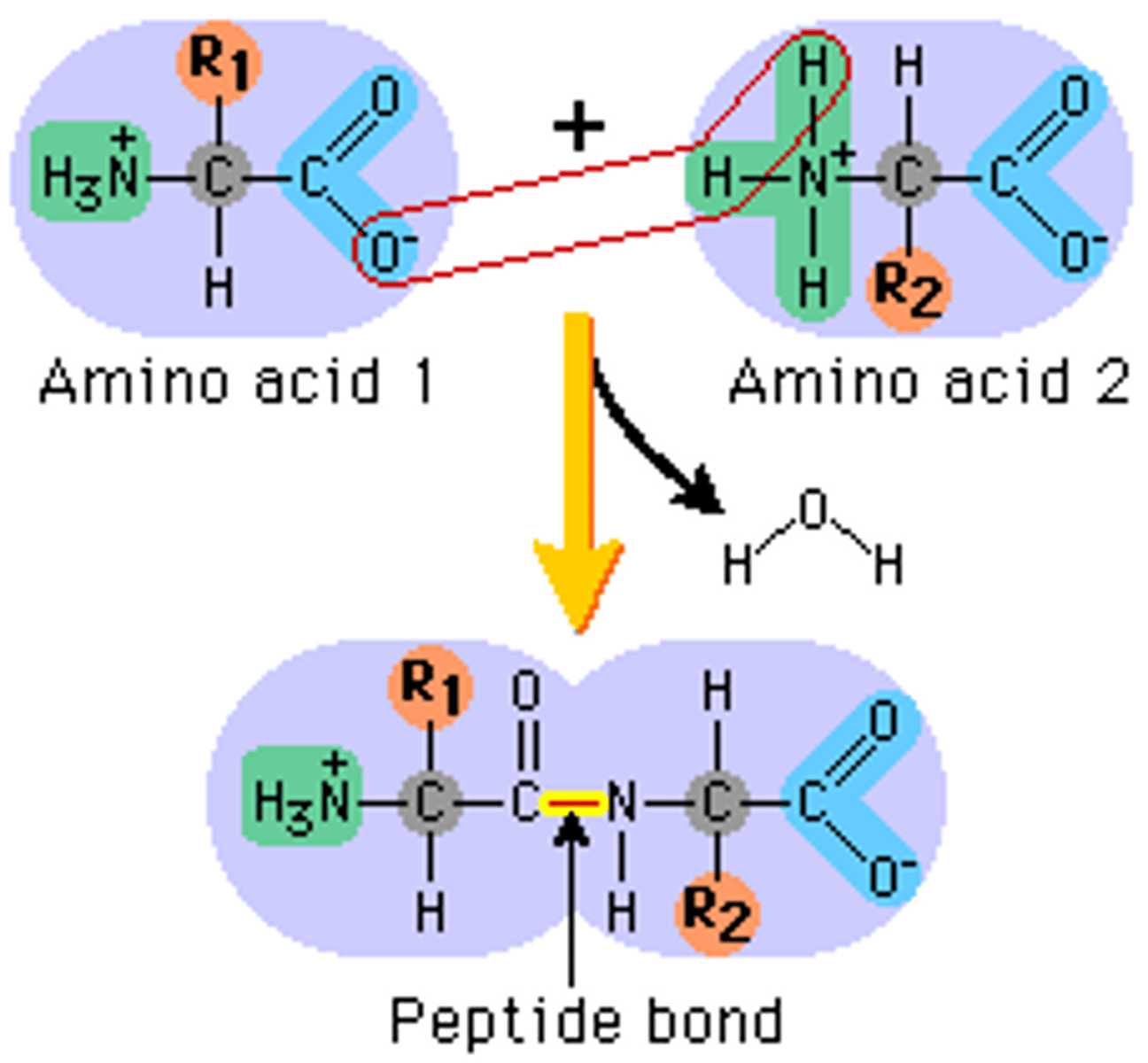
Peptide bond is an example of a _________________________ reaction.
condensation or dehydration. Why? Because peptide bond formation results in the removal of a water molecule (H2O).
What is the N-terminus?
When a peptide bond forms, the free amino end is known as the amino terminus (N terminus).
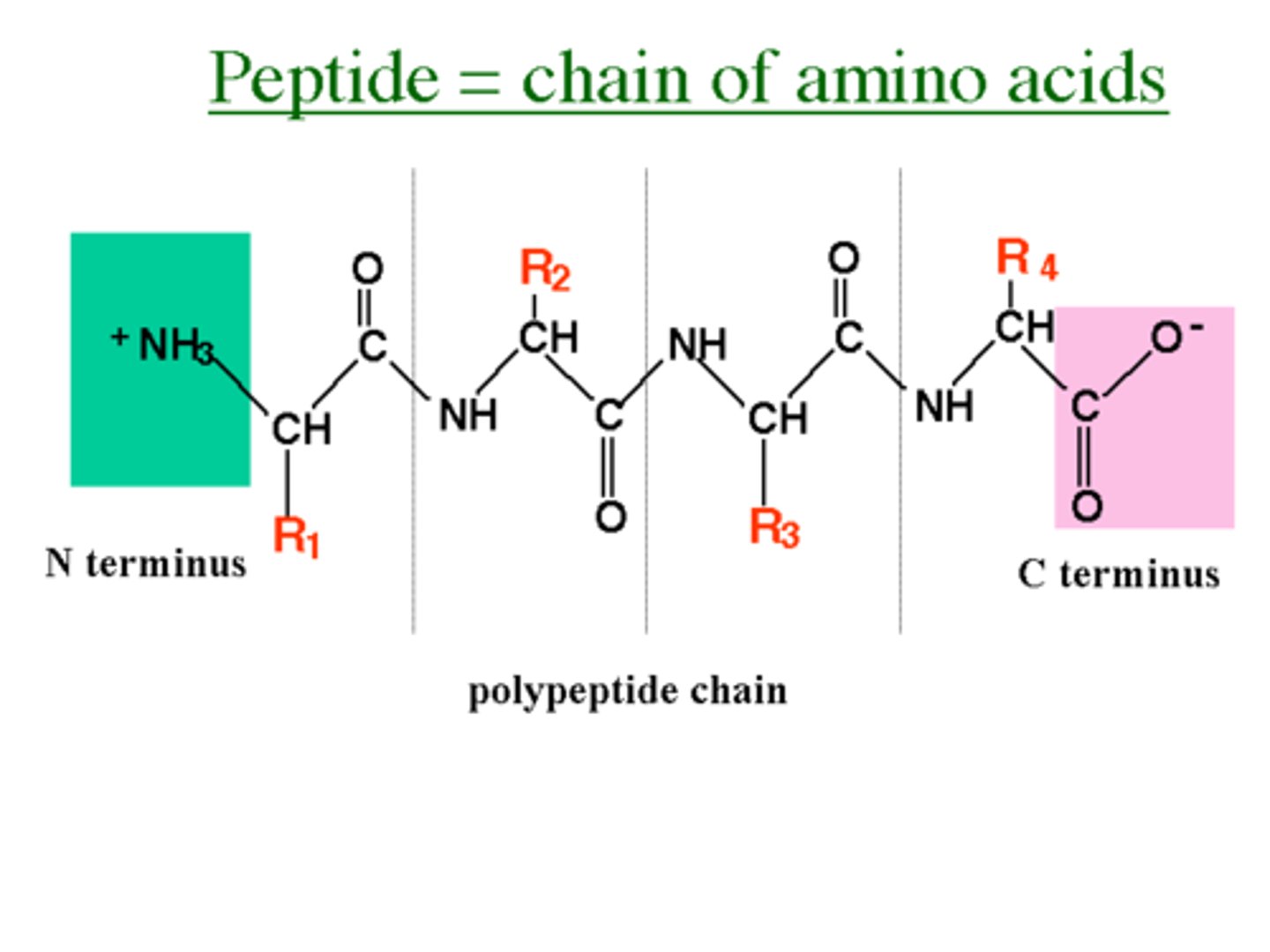
What is the C-terminus?
When a peptide bond forms, the free carboxyl end is known as the carboxy terminus (C-terminus).
How are peptides drawn and read?
By convention, peptides are drawn with the N-terminus on the left and the C-terminus on the right; similarly, they are read from N-terminus to C-terminus.
How are peptide bonds cleaved or polypeptides broken down?
Via hydrolysis which involves hydrolytic enzymes, such as trypsin and chymotrypsin. They break apart the amide bond by adding a hydrogen atom to the amide nitrogen and an OH group to the carbonyl carbon.
T/F: The reverse reaction, hydrolysis of the peptide bond, is catalyzed by a strong acid or base.
True.
Resonance in the Peptide Bond
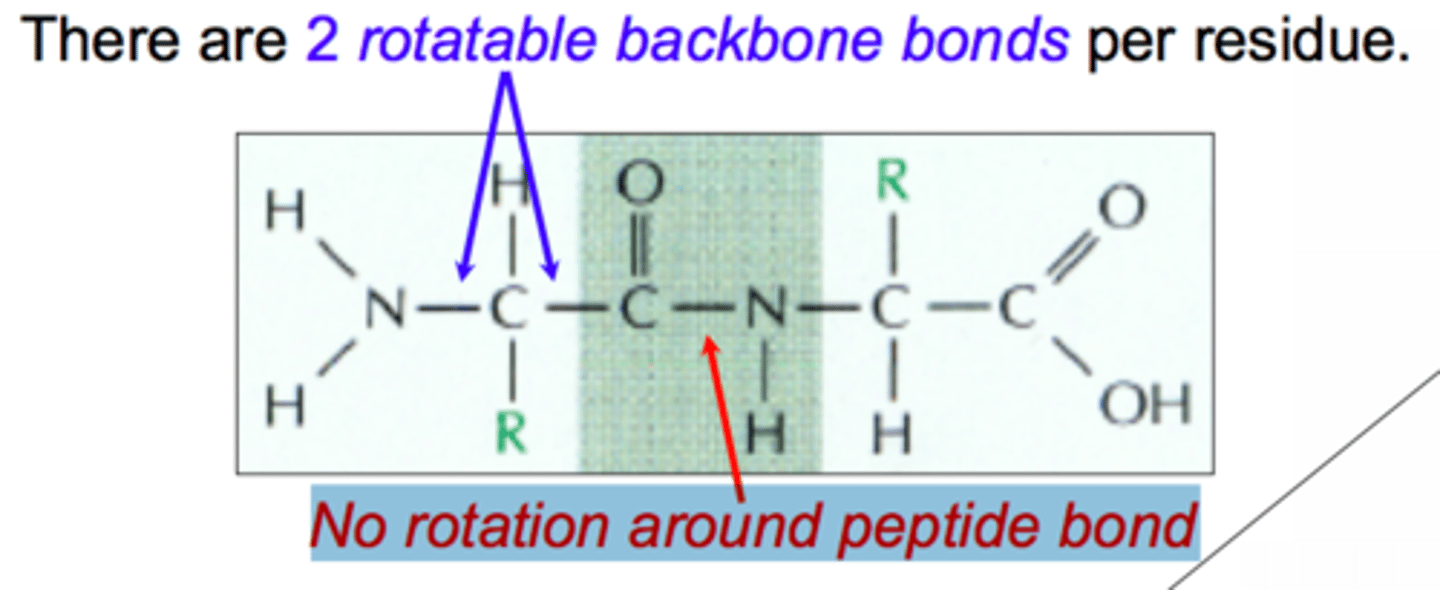
Because amide groups have delocalizable π electrons in the carbonyl and in the lone pair on the amino nitrogen, they can exhibit resonance; thus, the C-N bond in the amide has partial double bond character.
As a result, rotation of the protein backbone around its C-N amide bonds is restricted, which makes the protein more rigid. Rotation around the remaining bonds in the backbone, however, is not restricted, as those remain single (σ) bonds.
Extensive info: Like other carbonyl-containing functional groups, amides have two resonance structures, as shown in Figure 10.5. The true structure of the amide bond is therefore a hybrid of these two structures, with partial double-bond character between the nitrogen atom and the carbonyl carbon. This double-bond character limits rotation about the C-N bond, which adds to the rigidity and stability of the backbone of proteins. The single bonds on either side of the peptide bond, on the other hand, permit free rotation.
Characteristics of Peptide Bonds or Amide Bonds
- Covalent bond that joins two amino acids
- Dehydration synthesis: release of a water molecule
- Partial double bond character: shorter than a single bond.
- Rigid and planar: rotation around the bond is restricted. Rotation around the partial bond in the -CONH configuration is restricted, but rotation can occur with all other bonds. Thus rotation is restricted only in the peptide bond region.
- The bond is in trans configuration: The molecules attached are in different planes -- C=O is below the plane and N-H is above. The advantage of having trans configuration is the adjacent side chains in the bonded amino acids will have less steric hindrances.
- The peptide bond is uncharged. The side chains in the bonded amino acids may be charged: some may be basic/positively-charged -- Knights Riding Horses (KRH) refers to lysine, arginine, histidine. Some may be negatively charged -- Dragons Eat (DE) refers to aspartic acid and glutamic acid.
What are the four levels of structure for a protein?
1.) Primary
2.) Secondary
3.) Tertiary
4.) Quaternary
What is the primary structure of a protein?
The primary structure of a protein is the linear arrangement of amino acids coded in an organism's DNA. It's the sequence of amino acids, listed from the N-terminus, or amino end, to the C-terminus, or carboxyl end.
Primary structure is stabilized by the formation of covalent peptide bonds between adjacent amino acids..
The primary structure of a protein can be determined by a laboratory technique called _____________.
Sequencing.
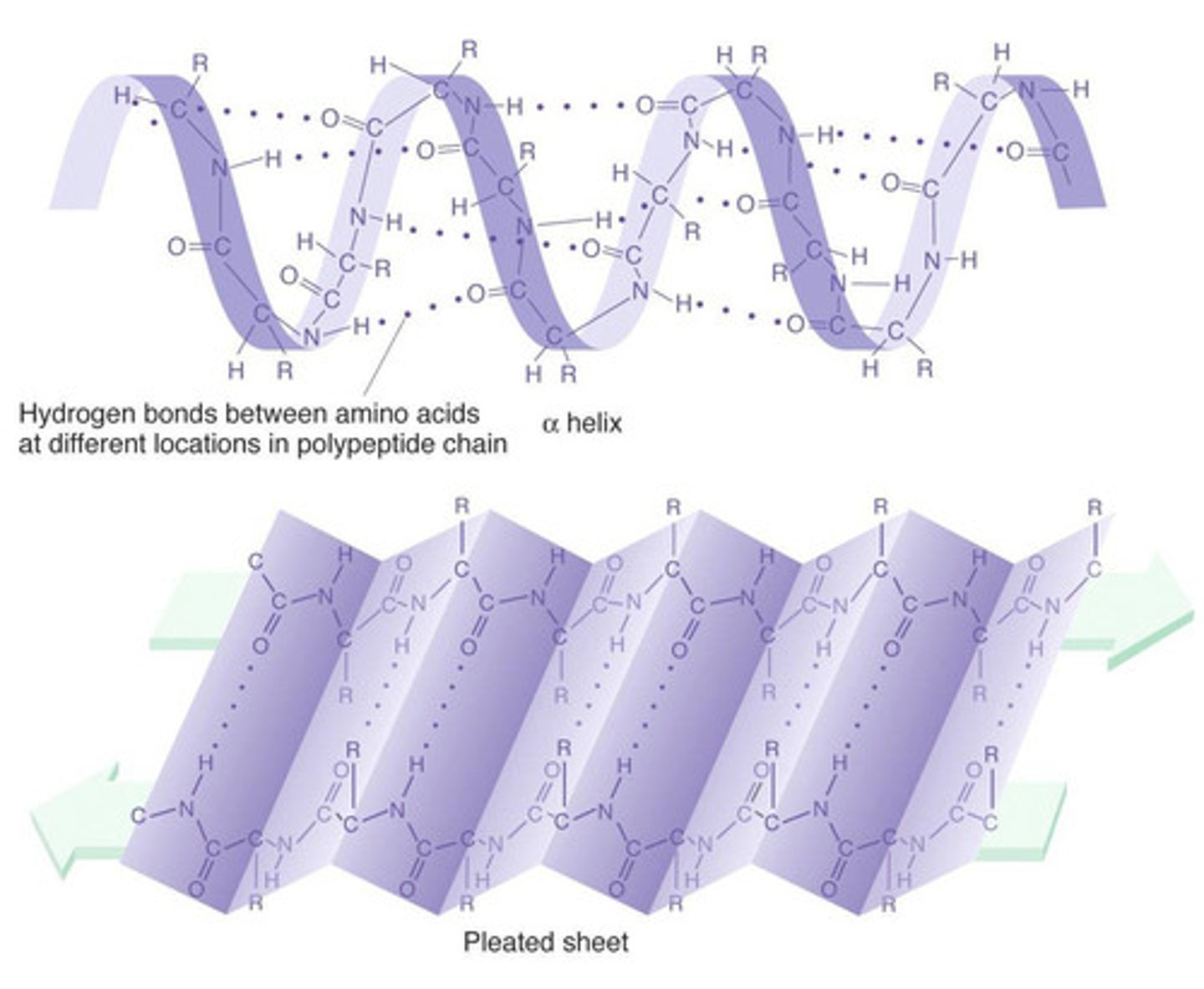
What is the secondary structure of a protein?
regular arrangement of amino acids within localized regions of the polypeptide. Secondary structures are primarily the result of hydrogen bonding between nearby amino acids.
What are the two most common secondary structures?
The two most common secondary structures are α-helices and β-pleated sheets. The key to the stability of both structures is the formation of intramolecular hydrogen bonds between different residues.
How can we distinguish between alpha helix and beta pleated sheets structures?
The side chains of the amino acids in the α-helical conformation point away from the helix core. The alpha helix is a rod-like structure whose inner section is formed by a tightly coiled main chain, with its side chains extending outward in a helical array.
To accommodate as many hydrogen bonds as possible, the β-pleated sheets assume a pleated, or rippled, shape. The R groups of amino residues point above and below the plane of the β-pleated sheet.
How is the alpha helix structure and keratin related?
The α-helix is an important component in the structure of keratin, a fibrous structural protein found in human skin, hair, and fingernails
How is the beta-pleated sheet structure and fibroin related?
Fibroin, the primary protein component of silk fibers, is composed of β-pleated sheets.
How does proline affect secondary structure?
Because of its rigid cyclic structure, proline will introduce a kink in the peptide chain when it is found in the middle of an α-helix. Proline residues are thus rarely found in α-helices, except in helices that cross the cell membrane.
Similarly, it is rarely found in the middle of pleated sheets. On the other hand, proline is often found in the turns between the chains of a β-pleated sheet, and it is often found as the residue at the start of an αhelix.
Proline acts as a structural disruptor in the middle of regular secondary structure elements such as alpha helices and beta sheets; however, proline is commonly found as the first residue of an alpha helix and also in the edge strands of beta sheets.
What is a protein's tertiary structure and what is it mostly determined by?
A protein's tertiary structure is its three-dimensional shape. Tertiary structures are mostly determined by hydrophilic and hydrophobic interactions between R groups of amino acids.
Hydrophobic residues prefer to be on the interior of proteins, which reduces their proximity to water. Hydrophilic N-H and C=O bonds found in the polypeptide chain get pulled in by these hydrophobic residues. These hydrophilic bonds can then form electrostatic interactions and hydrogen bonds that further stabilize the protein from the inside. As a result of these hydrophobic interactions, most of the amino acids on the surface of proteins have hydrophilic (polar or charged) R groups; highly hydrophobic R groups, such as phenylalanine, are almost never found on the surface of a protein
The tertiary structure of a protein is primarily the result of moving hydrophobic amino acid side chains into the interior of the protein.