SECTION 06: METABOLISM AND ENERGY MOLECULES
1/99
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
100 Terms
Q: What do DNA, RNA, and protein all require for their synthesis and regulation?
A: Energy.
Q: How do enzymes help with energy usage?
A: They make reactions more favorable and can couple reactions to the breakage of high-energy bonds.
Q: What is the most common high-energy molecule in the cell?
A: ATP (Adenosine Triphosphate).
Q: What are two other high-energy molecules used in cells?
A: NADH (Nicotinamide Adenine Dinucleotide) and FADH₂ (Flavin Adenine Dinucleotide).
Q: What is the role of ATP in metabolism?
A: Acts as the cell’s energy currency—used to power cellular processes.
Q: What will this section of the course explore?
A: How cells create and use energy through metabolic processes.
Q: What is metabolism?
A: An integrated network of chemical reactions where the product of one reaction becomes the substrate for the next.
Q: What molecules are involved in metabolic synthesis and breakdown?
A: Carbohydrates, lipids, and amino acids.
Q: What will you learn by the end of BCHM 270?
A: How metabolic pathways interact to provide energy and nutrients, and what happens when something goes wrong.
Q: What does Module 04 cover?
A: Carbohydrate metabolism:
Glycolysis (↓ arrows)
TCA cycle
Gluconeogenesis (↑ arrows)
Glycogen synthesis/degradation
Pentose phosphate pathway
Q: What does Module 05 focus on?
A: Lipid metabolism, including:
Energy storage
Cell membrane components
Hormones & signaling molecules
Q: What is covered in Module 06?
A: Metabolism of amino acids, urea, and nucleotides.
Q: What is homeostasis in metabolism?
A: It's the balance of metabolic activities to maintain stable nutrient and energy levels in the body.
Q: What does the figure with flasks and tubes represent?
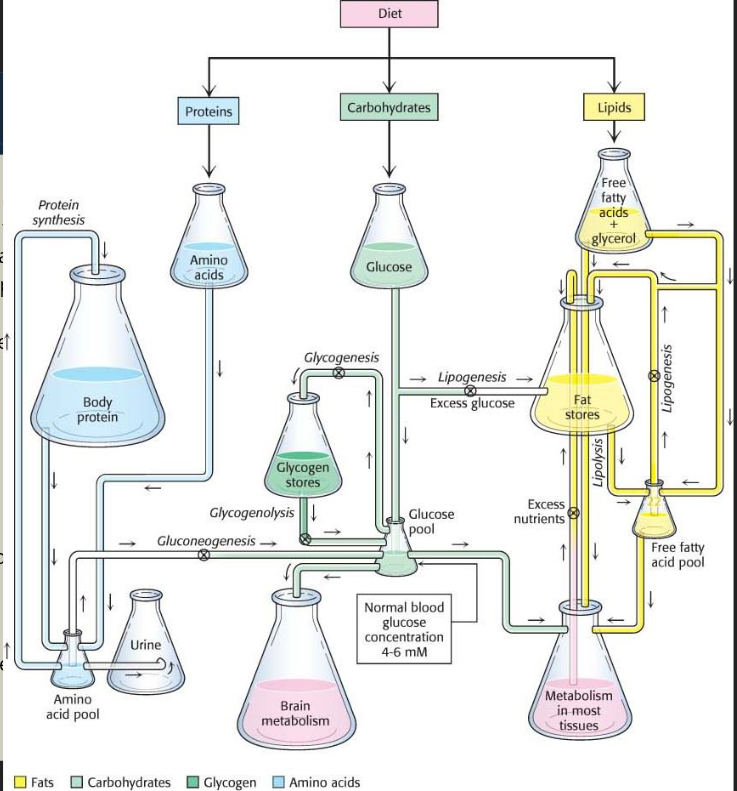
A: The interconnected pathways that maintain nutrient balance between proteins, carbs, lipids, and tissues.
Q: What are the 3 main ways metabolic processes are regulated?
Amount of enzymes (controlled by synthesis/degradation)
Enzyme activity (controlled by cofactors, allosteric regulation, covalent modification, etc.)
Substrate accessibility (controlled by compartmentalization)
Q: What is an example of substrate accessibility?
A: Opposing reactions like fat breakdown vs. fat synthesis occur in different places (compartments) to prevent interference.
Q: What is the goal of all these regulations?
A: To keep blood glucose stable and ensure each tissue gets the right fuel at the right time.
Q: Where is DNA & RNA made in the cell?
A: In the nucleus – the cell’s genetic library.
Q: Which metabolic processes happen in the cytosol?
A:
Glycolysis
Pentose phosphate pathway
Fatty acid synthesis
Protein synthesis
Q: What do proteosomes do and where are they found?
A: They’re in the cytosol and break down damaged proteins into amino acids.
Q: Which processes occur in the mitochondria?
A:
TCA cycle
Electron transport chain (ETC)
ATP production via oxidative phosphorylation
Beta-oxidation of fatty acids
Q: What happens in the Golgi apparatus?
A: Glycosylation of proteins (adding sugar chains to proteins).
Q: What’s the role of the endoplasmic reticulum (ER)?
A:
Rough ER: Protein synthesis
Smooth ER: Long-chain fatty acid synthesis
Q: What is the function of the lysosome?
A: It breaks down large or damaged structures (e.g., organelles, proteins, DNA) into basic building blocks.
Q: What is cellular metabolism?
A: It's the sum of all reactions in a cell that build or break down molecules.
Q: What are the two main types of metabolic reactions?
A:
Anabolism
Catabolism
Q: What is anabolism?
A:
Builds larger molecules from smaller ones
Uses ATP
Example: Gluconeogenesis (makes glucose from smaller molecules)
Q: What is catabolism?
A:
Breaks down large molecules (e.g., carbs, fats)
Releases energy
Energy is stored as ATP
First example you’ll study: Glycolysis
Q: What is metabolism?
A: Metabolism is all the chemical reactions in your body. It includes two opposite processes: anabolism (building molecules) and catabolism (breaking molecules down).
Q: What are anabolic reactions?
A: Anabolic reactions build large molecules from small ones (like proteins from amino acids) and use energy (ATP).
Q: What are catabolic reactions?
A: Catabolic reactions break down large molecules (like glucose or fat) into smaller ones and release energy, which is stored in ATP.
Q: What is ATP and why is it important?
A: ATP is the main energy currency of the cell. It powers all processes that require energy, like muscle contraction or protein synthesis.
Q: What are the six essential nutrients your body needs?
A: Water, vitamins, minerals, carbohydrates, lipids (fats), and proteins.
Q: What’s the main fuel your body uses for energy?
A: Glucose (a simple carbohydrate). It's critical for the brain and red blood cells.
Q: What happens to carbs if they’re not used right away?
A: They get stored as glycogen (short-term) or converted into fat (long-term storage).
Q: Why are fats important (besides storing energy)?
A: Fats cushion organs, store fat-soluble vitamins, insulate nerves (myelin), and are used to build hormones and cell membranes.
Q: What are essential fatty acids?
A: Fats your body can’t make (like omega-3 and omega-6). You must get them from food.
Q: What do proteins do in the body?
A: Proteins make up muscles, enzymes, cell channels, pumps, and many other functional molecules.
Q: Can the body make all the amino acids it needs?
A: No. There are 9 essential amino acids you must get from your diet.
Q: What’s the relationship between food and the body?
A: Your body constantly breaks food down into building blocks, then rebuilds those blocks into new body parts — like proteins and cell membranes.
Q: What are the 4 main tissues involved in energy metabolism?
A: Liver, adipose (fat), skeletal muscle, and brain.
Q: What does each metabolic tissue do?
Liver: Makes, stores, and releases energy sources
Adipose: Stores fat and releases fatty acids
Muscle: Uses glucose and fat for movement
Brain: Uses glucose (mostly) for energy
Q: How do these tissues interact?
A: They form a network and share energy molecules (substrates).
Q: What controls the coordination between tissues?
A:
Hormones (especially insulin and glucagon)
Nervous system signals
Levels of circulating substrates in the blood
Q: What triggers hormone release for metabolic control?
A: Changes in blood energy levels — usually depending on when you last ate.
Q: What two main hormones control energy metabolism?
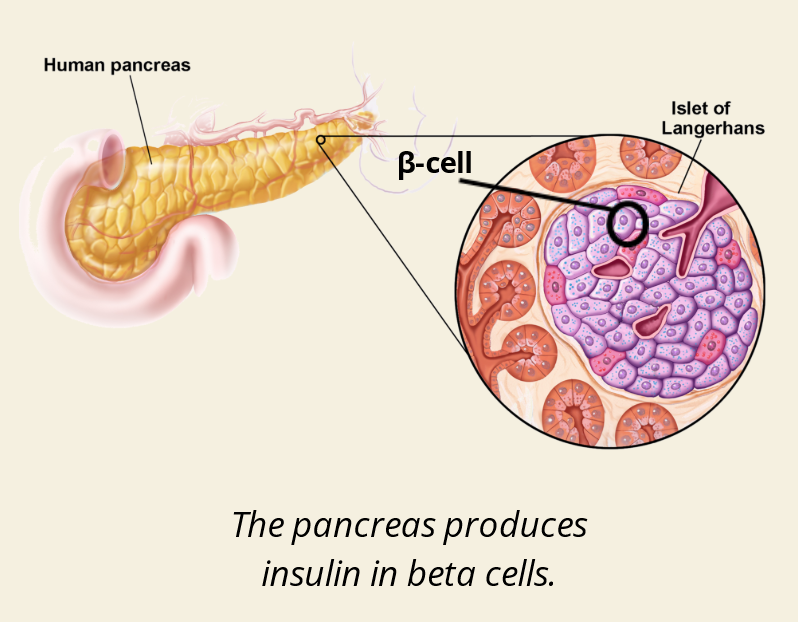
A: Insulin and Glucagon.
Q: Where is insulin made?
A: In β-cells (beta cells) of the pancreas, specifically in the Islets of Langerhans.
Q: When is insulin released and what does it do?
Released after a meal when energy levels are high.
Anabolic hormone → builds and stores:
Increases synthesis of proteins, fats, and carbs
Promotes energy storage
Q: When is glucagon released and what does it do?
A:
Released when energy is low (fasting or low blood sugar).
Catabolic hormone → breaks down stored molecules to release energy
Maintains blood glucose levels
Q: What other hormones help during stress or danger?
A: Catecholamines like epinephrine and norepinephrine (fight or flight response).
Q: What will be explored in the next modules of BCHM 270?
A: Major metabolic pathways and their regulation by key hormones.
Q: What kind of enzymes are regulated by hormones like insulin and glucagon?
A: Allosteric enzymes at key control points in metabolic pathways.
Q: Which hormones commonly regulate metabolic enzymes?
A:
Insulin
Glucagon
(Also epinephrine, to a lesser extent)
Q: Why are these hormonal controls important?
A: They determine whether a pathway is activated or inhibited based on the body’s energy state.
Q: What are the main energy “currencies” used in metabolism?
A:
ATP (adenosine triphosphate)
GTP (guanosine triphosphate)
NADH
FADH₂
NADPH
Q: What is ATP made of?
A: Adenosine + Ribose sugar + 3 phosphate groups
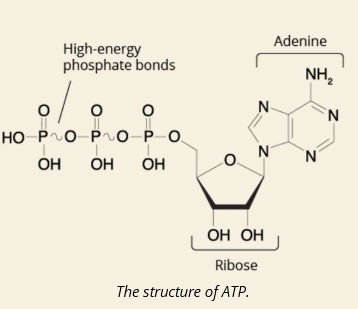
Q: What is the role of ATP in the cell?
A: It's the main energy source for most cellular processes.
Q: What is GTP and how is it different from ATP?
A: GTP is like ATP, but it has guanosine instead of adenosine.
Q: What are some functions of GTP?
A:
Powers protein synthesis (peptide bond formation)
Fuels gluconeogenesis
Acts as backup energy when ATP is low
Q: When does the cell use GTP instead of ATP?
A: In specific reactions or energy-poor conditions
Q: What happens when ATP loses its 3rd phosphate group?
A: It becomes ADP and releases energy (~–7.3 kcal/mol).
Q: What happens when ADP loses a phosphate?
A: It becomes AMP and releases more energy (~–7.3 kcal/mol again).
Q: Why is ATP called a high energy molecule?
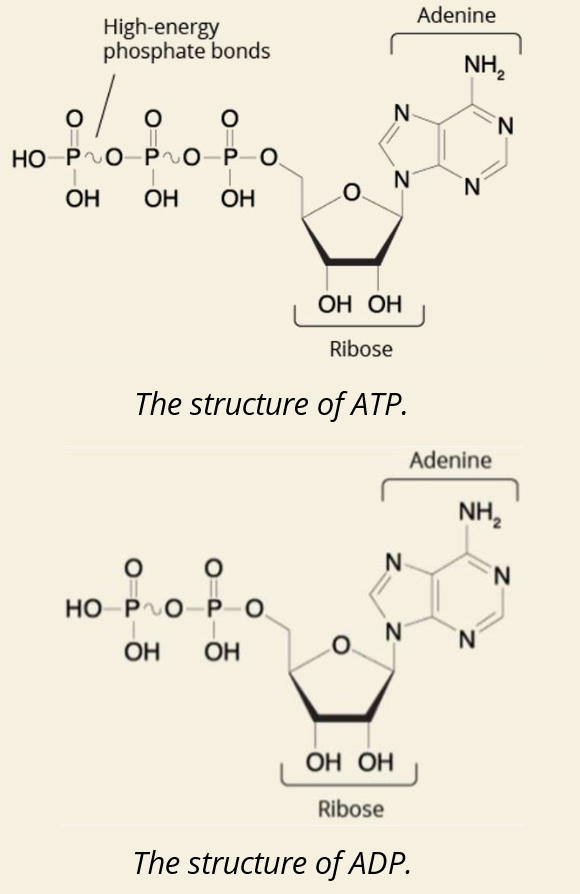
A: Because the bonds between phosphate groups store a lot of free energy (ΔG° = –7.3 kcal/mol per bond broken).
Q: What does Pi mean in biology?
A: Pi stands for inorganic phosphate (PO₄³⁻), released when ATP is broken down.
Q: What is Standard Free Energy (ΔG°)?
A: It’s the change in energy under standard conditions:
Temp = 273.15 K
Pressure = 1 atm
Q: Why is ATP called the "energy currency" of the cell?
A: Because it stores energy in its phosphate bonds and transfers that energy to power other reactions.
Q: How do enzymes use ATP to drive reactions forward?
A: Enzymes often transfer a phosphate group from ATP to the substrate or to themselves, which helps power the next step of the reaction.
Q: What happens when ATP is used in a reaction?
A: A phosphate is removed, releasing free energy (ΔG°) that pushes the reaction forward.
Q: What is a common intermediate step when ATP is used?
A: ATP forms a phosphorylated intermediate, which is more reactive and helps the reaction proceed.
Q: Can other molecules act like ATP?
A: Yes — molecules like GTP, NADH, FADH₂ also store and transfer energy in the cell.
Q: What is energy coupling in metabolism?
A: It’s when an unfavourable (endergonic) reaction is paired with a favourable (exergonic) one to make the overall process happen.
Q: Why do we need energy coupling?
A: Some important reactions (like protein synthesis) require energy and can’t happen on their own — they need help from reactions that release energy, like ATP hydrolysis.

Q: What is a common intermediate in energy coupling?
A: A molecule that is the product of one reaction and the reactant of the next, like D in the example.
Q: How does a common intermediate help with energy coupling?
A: It links two reactions, allowing the energy from the first (exergonic) to drive the second (endergonic).
Q: In this example, what is the common intermediate?

A: D is the common intermediate — it is made in Reaction 1 and used in Reaction 2.
🔁 Energy Coupling — ATP Drives Unfavorable Reactions

⚗ Example: Glutamine Synthetase Reaction

⚙ Allosteric Regulation by ATP
Q: What is an allosteric factor?
A: A molecule that binds to an enzyme outside the active site and changes the enzyme's shape and activity.
Q: How does ATP act as an allosteric factor?
A: ATP binds to certain enzymes and changes their shape → this can either activate or inhibit the enzyme's function depending on the enzyme.
Q: What happens if ATP is hydrolyzed to ADP?
A: The shape of the enzyme changes again, further modifying activity.
🔄 Sodium-Potassium Pump Example (You don’t need to memorize steps)
Q: What is the sodium-potassium pump?
A: An active transport protein that uses ATP to move ions across the cell membrane.
soddium potassium poump - Q: What ions are moved and where?
A:
3 Na⁺ (sodium) out of the cell
2 K⁺ (potassium) into the cell
sodium potassium pump - Q: How does ATP help?
3 Na⁺ bind to the pump (inside cell)
ATP is used → phosphate attaches to the pump
This changes the pump shape → releases Na⁺ outside
2 K⁺ bind from outside
Phosphate leaves → pump changes shape again
K⁺ released inside the cell
sodium potasssium pump - Q: What’s the key takeaway?
A: ATP powers the pump by binding and causing shape changes—this is allosteric regulation in action!
Q: What are two key high-energy electron carriers in the cell (besides ATP/GTP)?
A: NADH and FADH₂.
🧪 FAD (Flavin Adenine Dinucleotide)
Q: What reaction turns FAD into its high-energy form?
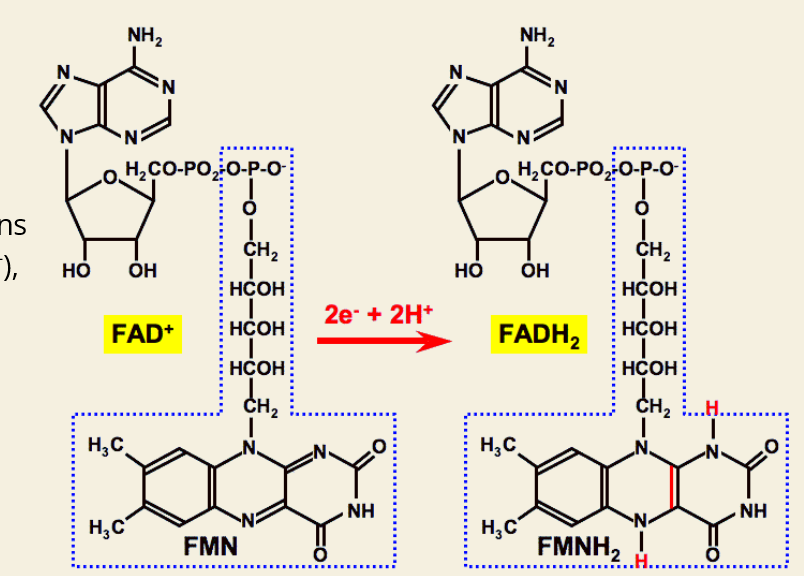
A: FAD + 2 e⁻ + 2 H⁺ → FADH₂
Q: What vitamin is used to make FAD?
A: Vitamin B2 (Riboflavin)
Q: What is FMN?
A: Flavin Mononucleotide, an intermediate made from B2 that can also help store energy.
⚡ NAD⁺ (Nicotinamide Adenine Dinucleotide)
Q: What reaction turns NAD⁺ into its high-energy form?
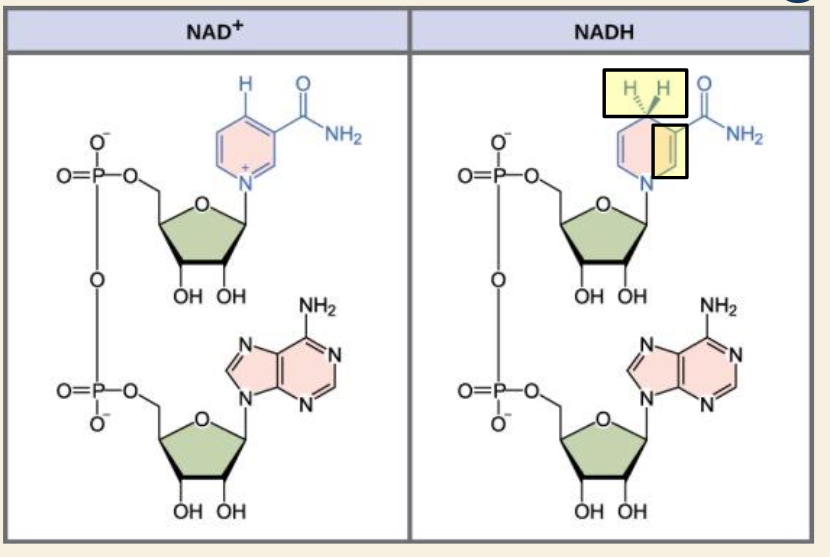
A: NAD⁺ + 2 e⁻ + H⁺ → NADH
Q: What vitamin is used to make NAD⁺/NADH?
A: Vitamin B3 (Niacin)
Q: What is NADPH used for?
A: NADPH is used in anabolic (building) reactions like gluconeogenesis and lipid synthesis.
Q: How is NADP⁺ different from NAD⁺?
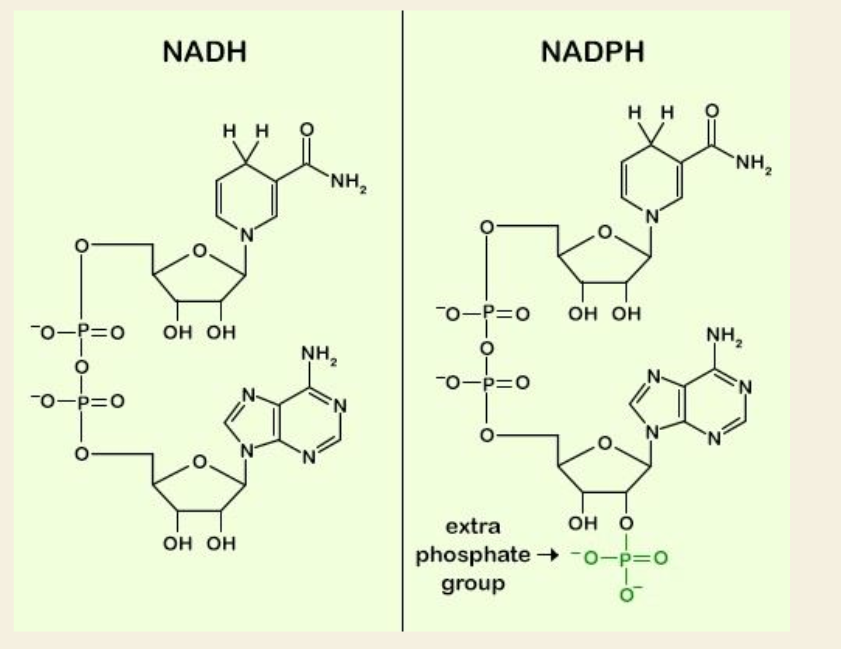
A: NADP⁺ has one extra phosphate group (highlighted in green in the diagram).
Q: Does the extra phosphate on NADP⁺ affect the reaction chemistry?
A: No — it doesn’t change the redox chemistry but does affect enzyme binding.
Q: Why do cells use NADPH instead of NADH for some reactions?
A: The extra phosphate makes NADPH bind different enzymes that are specific to biosynthesis pathways.
Q: What do catabolic reactions do?
A: Break down molecules to release energy and produce ATP, NADH, FADH₂
Q: What do anabolic reactions do?
A: Build larger molecules and use energy (require ATP, NADPH, etc.)
Q: Which hormone triggers anabolism after a meal?
A: Insulin – promotes storage and synthesis when energy is high.
Q: Which hormones trigger catabolism when energy is low?
A: Glucagon and epinephrine – stimulate breakdown of stored fuel like fat.
Q: Why are NADPH and GTP important?
A: They support special energy-demanding reactions, especially when ATP is low or other pathways are active.
Q: What major processes will be studied next to understand ATP production?
A: Cellular respiration, TCA (Krebs) cycle, and oxidative phosphorylation.