Chem A-Level PPQs
0.0(0)
Card Sorting
1/32
There's no tags or description
Looks like no tags are added yet.
Last updated 5:34 PM on 1/18/26
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
33 Terms
1
New cards


2
New cards

3
New cards

4
New cards

5
New cards


6
New cards


7
New cards


8
New cards
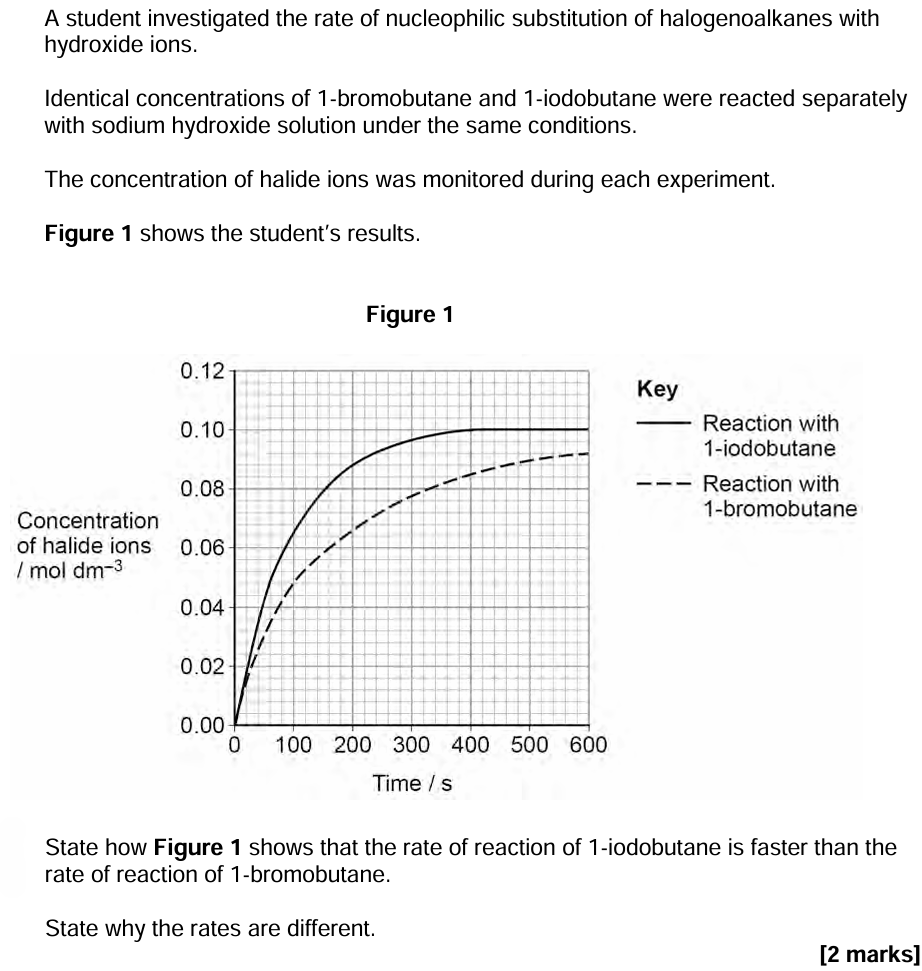
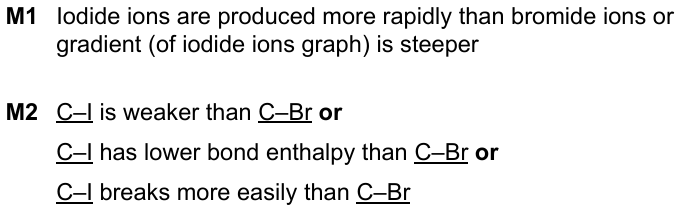
9
New cards
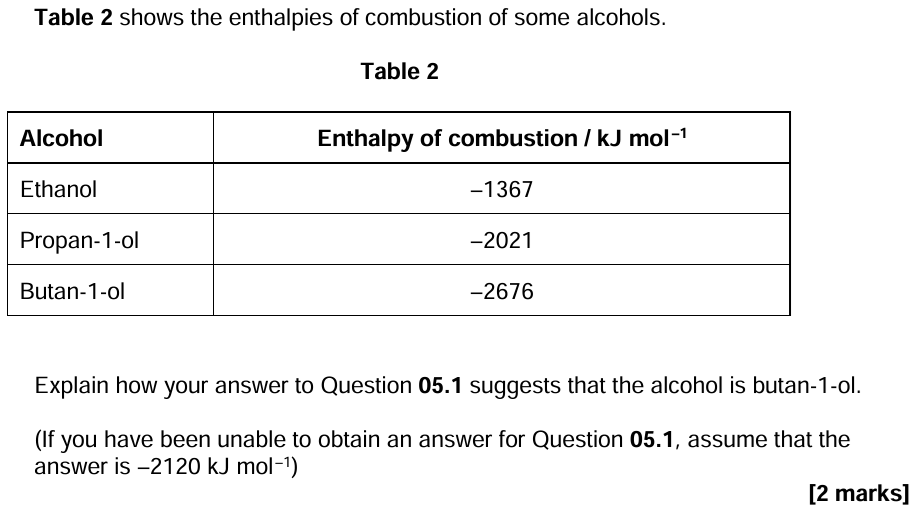

10
New cards


11
New cards
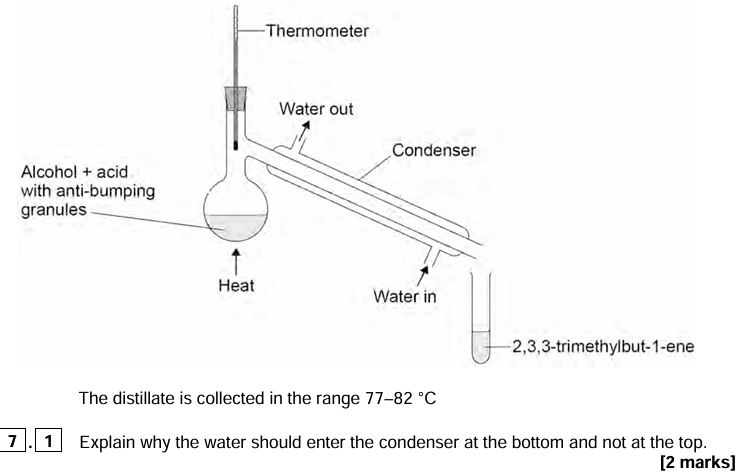

12
New cards
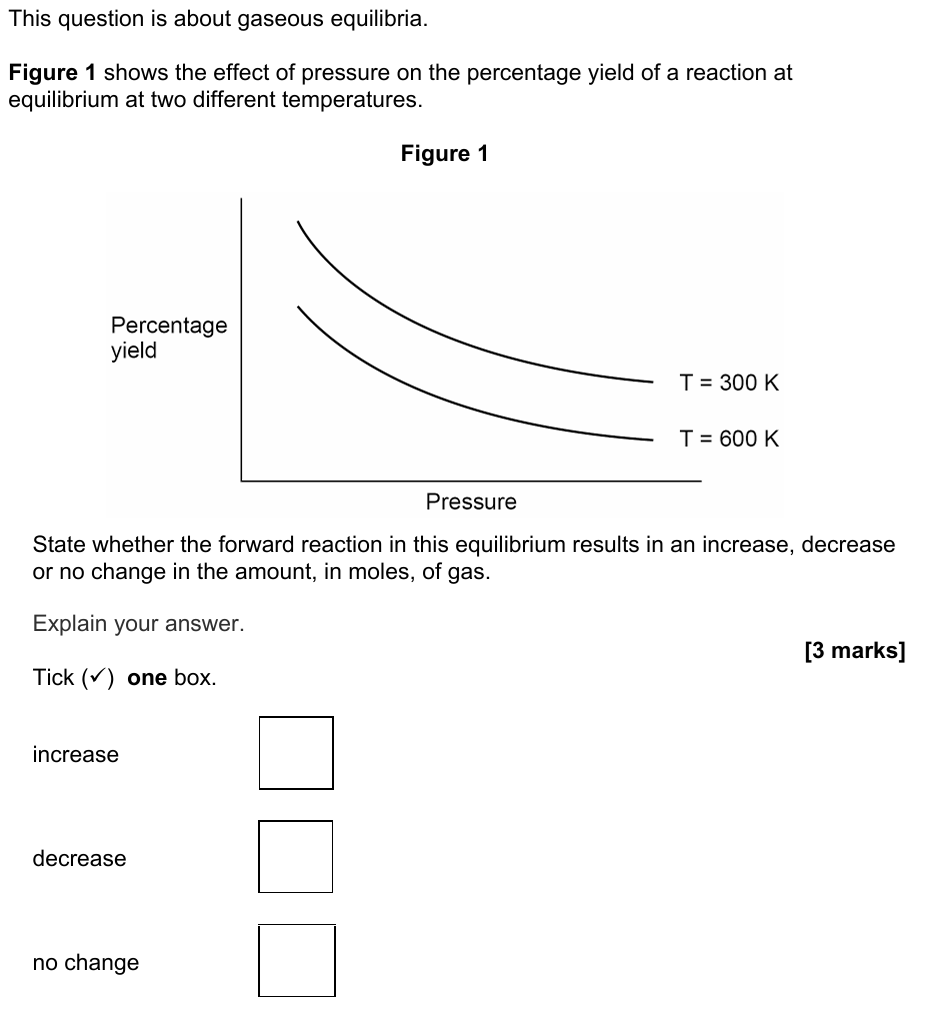

13
New cards
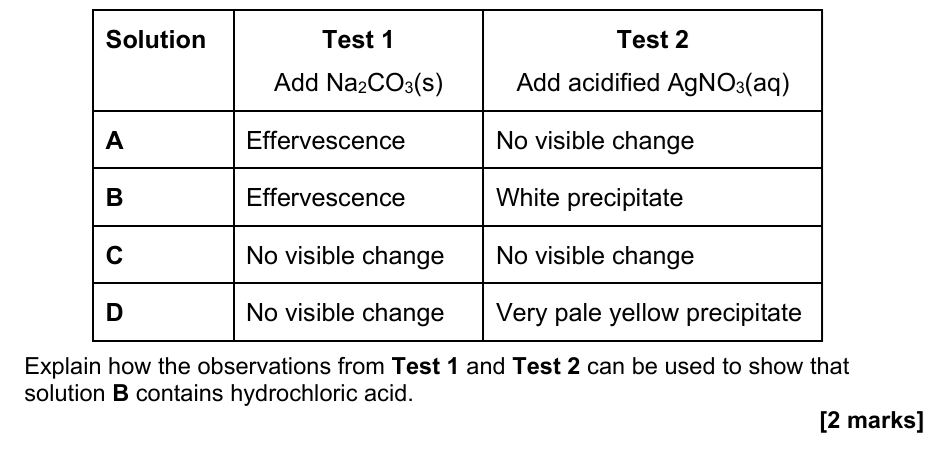

14
New cards

(can’t say red litmus dipped in solution)
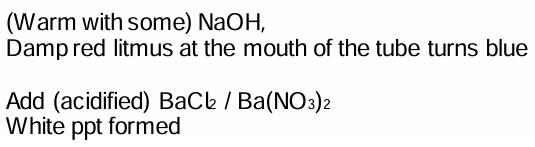
15
New cards
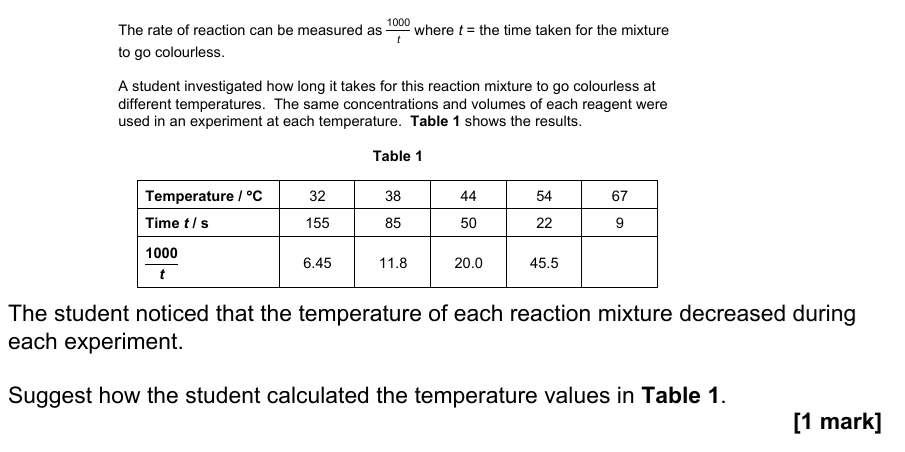

16
New cards


17
New cards
Tests for Grp2, Ammonium and Sulfates

18
New cards
Tests for hydroxides, halides and carbonates
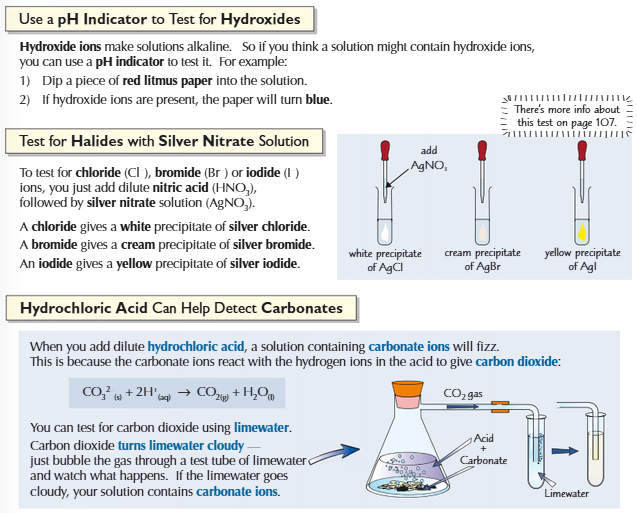
19
New cards
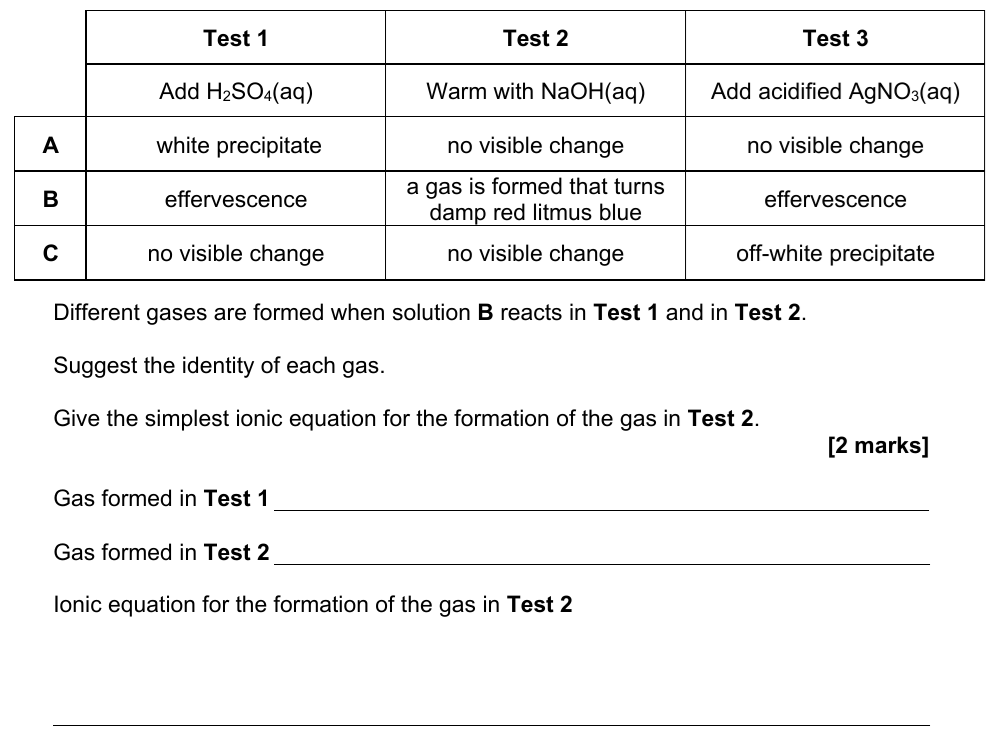
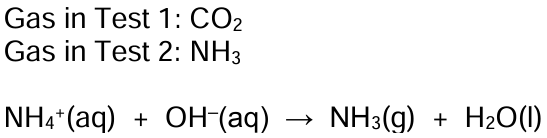
20
New cards


21
New cards

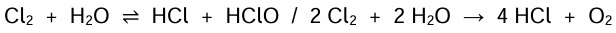
22
New cards

23
New cards
24
New cards
25
New cards

26
New cards

27
New cards

28
New cards


29
New cards


30
New cards


31
New cards


32
New cards
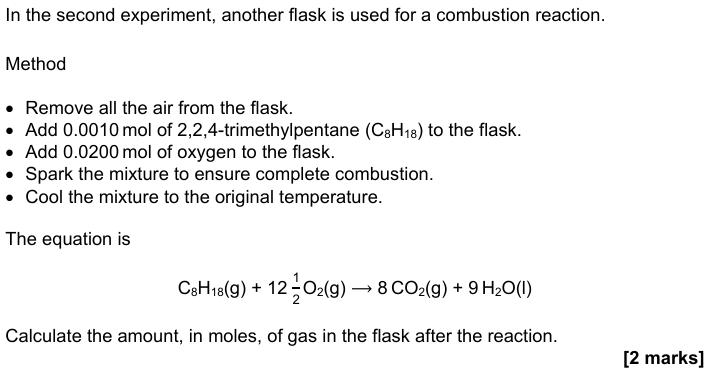
1 mol C8H18 needs 12.5 mol of O2
So, 0.0010 mol C8H18 needs: 0.0010×12.5 = 0.0125mol O2
There is 0.0200mol O2 available, meaning O2 is in excess, and C8H18 is limiting
CO2 produced = 0.0010 × 8 = 0.0080mol
O2 used = 0.0125mol
O2 remaining = 0.0200-0.0125 = 0.0075mol
Total gas = CO2 + leftover O2 = 0.0080 + 0.0075 = 0.0155mol

33
New cards