Gases in the atmosphere
1/22
Earn XP
Description and Tags
chem
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
water
formula
%
properties
uses
H2O
varies
polar molecule
required by living creatures
uses of carbon dioxide + why (property)
fizzy drinks - soluble in water
fire extinguisher - denser than air, doesn't support combustion
refridgerant - dry ice, solid co2, sublimes so no wet cardboard
Unburned fuels
how formed
problems
solutions
incomplete combustion
breathing problems, less efficient
ensure correct fuel:air mixture when fuel is burned
test for CO2
bubble through limewater
solution will go from colourless to cloudy
sulfur + oxygen
word equation
basic or acidic
what do you see
sulfur + oxygen ---> sulfur dioxide
acidic
glows blue
sodium+ oxygen
word equation
basic or acidic
what do you see
sodium + oxygen ----> sodium oxide
basic
yellow flame + white smoke
SO4 sulfur dioxide
how formed
problems
solutions
sulfur impurity burns with fossil fuels
acidic rain: damages plants, kills fish, corrodes limestone
remove SO4 by flue gas desulfurisation
phosphorous to find percentage of oxygen in air
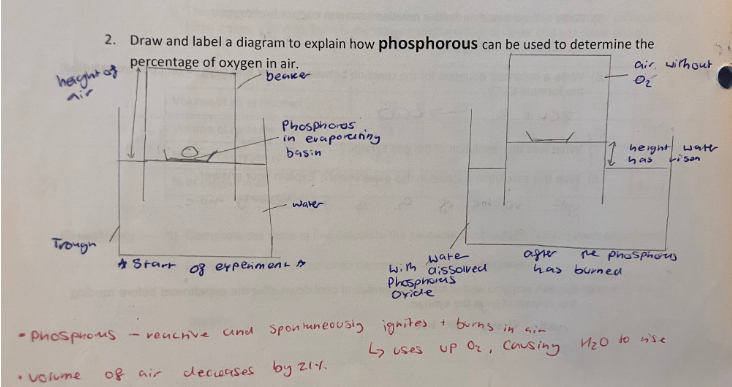
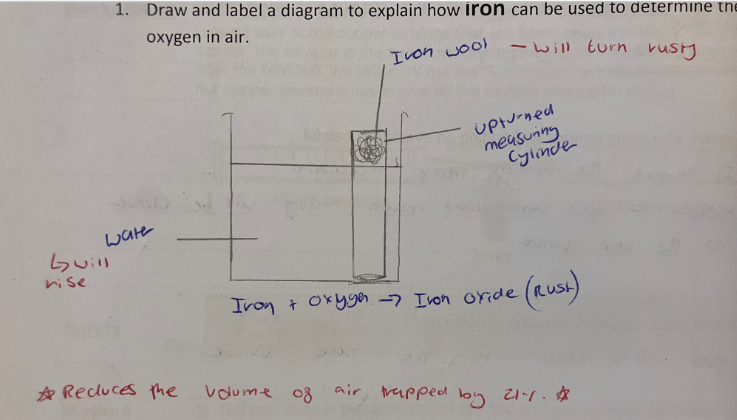
percentage of oxygen in the air using iron
oxygen
formula
%
properties
uses
O2
20%
very reactive, required for respiration
hospitals - patient breathing
NO/NO2 Nitrogen oxides
how formed
problems
solutions
car engines - N2 in air is reacting at high temos
acid rain
catalytic converters
Nitrogen
formula
%
properties
uses
N2
78%
unreactive
food packets
melting point of CO2 and explanation
low - weak intermolecular forces between molecules
co2 is simple molecular bonding
Magnesium + oxygen
word equation
basic or acidic
what do you see
magnesium + oxygen ----> magnesium oxide
basic
bright white light + white powder
is co2 soluble in water?
yes
in general, metal oxides are _____ and non-metal oxides are ____
basic, acidic
CO2 - carbon dioxide
how formed
problems
solutions
complete combustion of c in fuel
greenhouse gas causing global warming
burn less fossil fuels
CO - Carbon monoxide
how formed
problems
solutions
incomplete combustion of C in fuel
TOXIC
ensure good supply of air/oxygen when burned
carbon dioxide
formula
%
properties
uses
CO2
0.03%
unreactive
fire extinguishers
carbon + oxygen
word equation
basic or acidic
what do you see
carbon + oxygen -----> carbon dioxide
acidic
glows red
C soot/particulates
how formed
problems
solutions
incomplete combustion of C in fuel
blackens buildings, global dimming, breathing issues
ensure there is a good supply of oxygen/air when burned
Argon
formula
%
properties
uses
Ar
0.9%
very unreactive
inside filament lightbulbs
appearance of carbon dioxide
colourless gas