Periodic table: symbol + name, group names + locations, Greek prefixes for naming covalent compounds
1/66
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
67 Terms
N
Nitrogen
Ti
Titanium
Cr
Chromium
Mn
Manganese
Fe
Iron
Co
Cobalt
Ni
Nickel
Cu
Copper
Zn
Zinc
As
Arsenic
Br
Bromine
Kr
Krypton
Rb
Rubidium
Sr
Strontium
Ag
Silver
Sn
Tin
I
Iodine
Xe
Xenon
Cs
Cesium
Ba
Barium
W
Tungsten
Pt
Platinum
Au
Gold
Hg
Mercury
Pb
Lead
Bi
Bismuth
Rn
Radon
La
Lanthanum
Ac
Actinium
U
Uranium
Pu
Plutonium
Alkali metals
Any metal in Group 1A of the periodic table
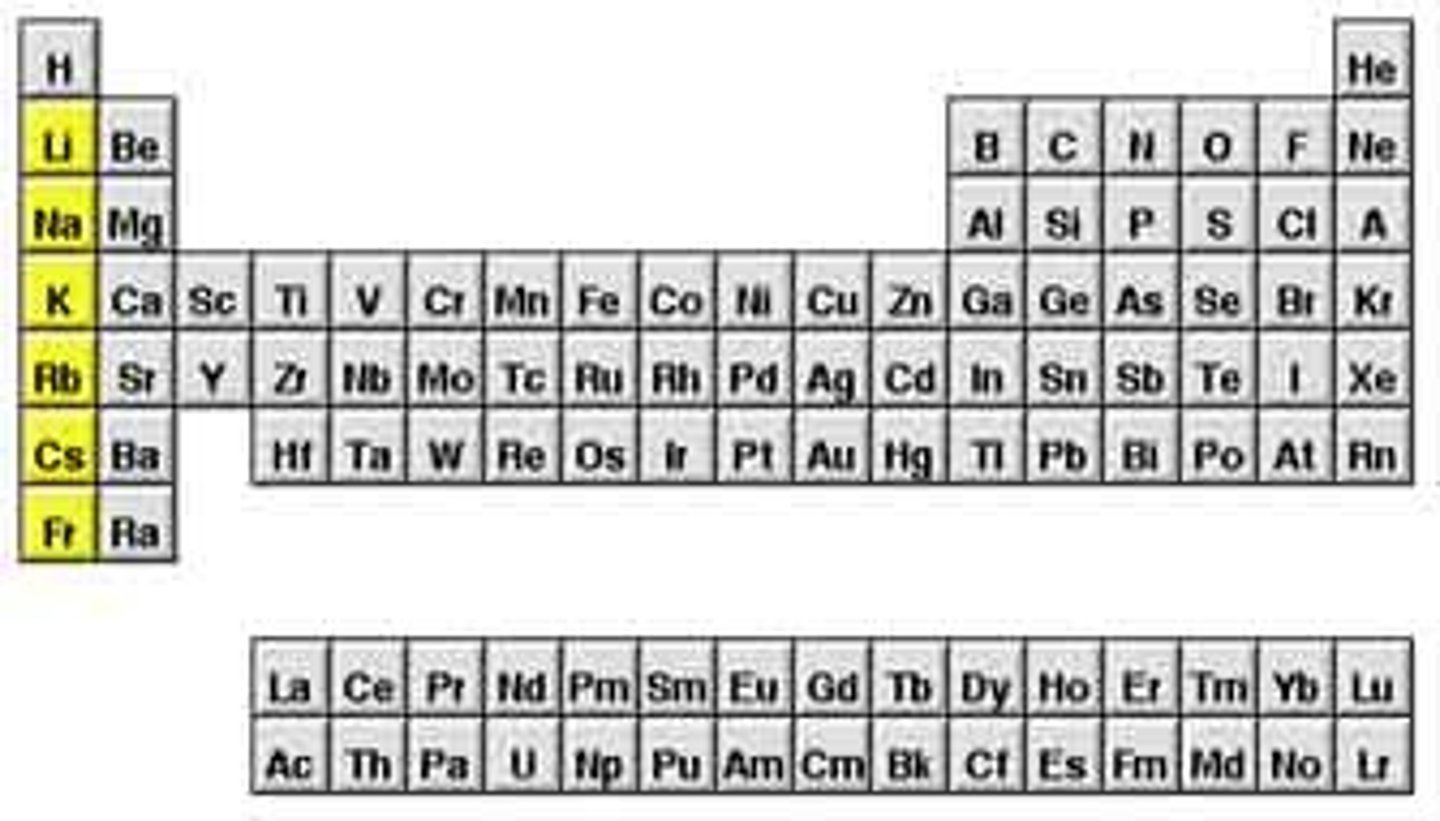
Alkaline Earth metals
metallic elements in group 2 of the periodic table which are harder than the alkali metals and are also less reactive
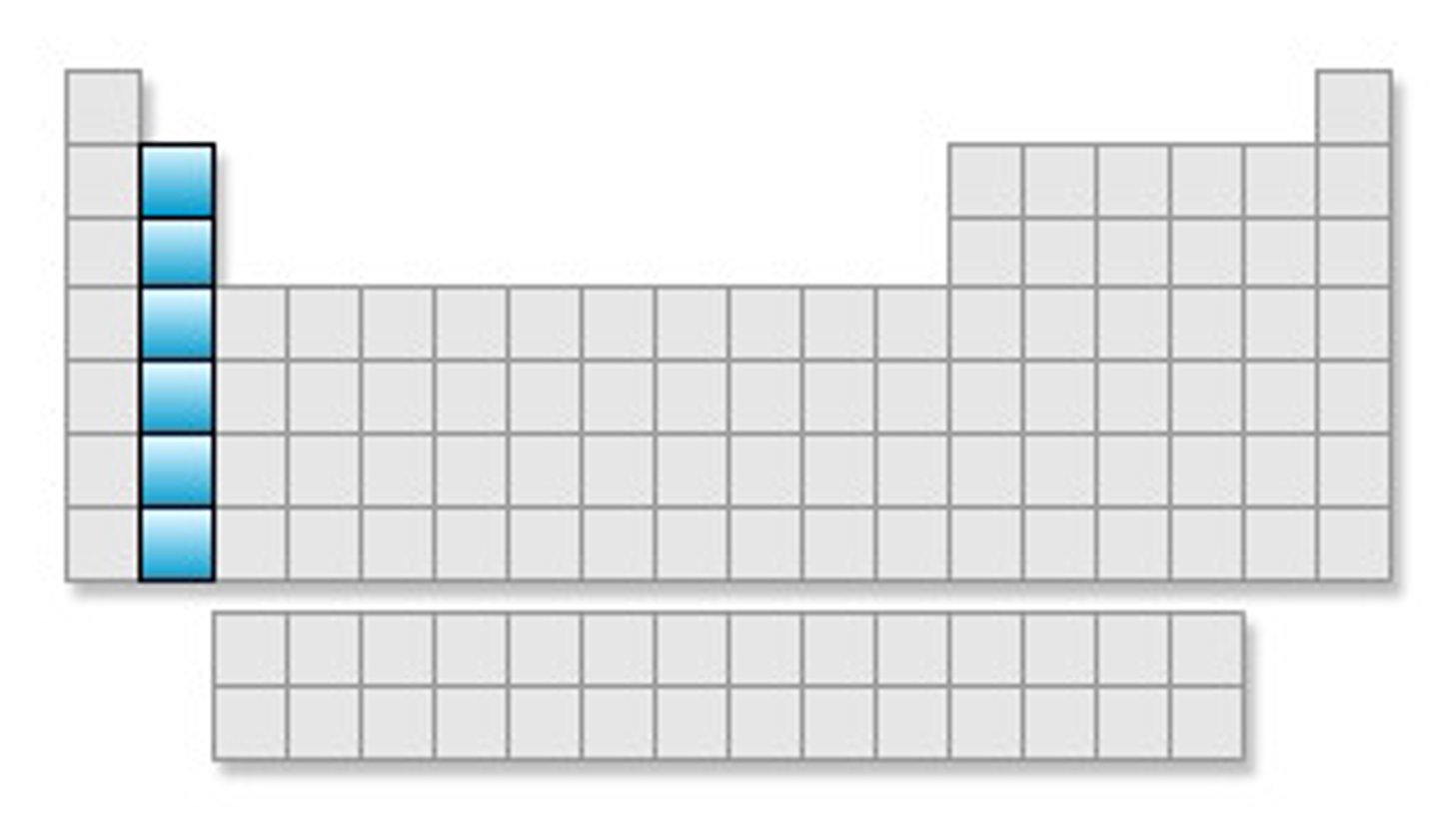
Transition metals
Groups 3-12, 1-2 electrons in the outer energy level, less reactive than alsali-earth metals, shiny, good conductor of thermal energy and electrical current, high density
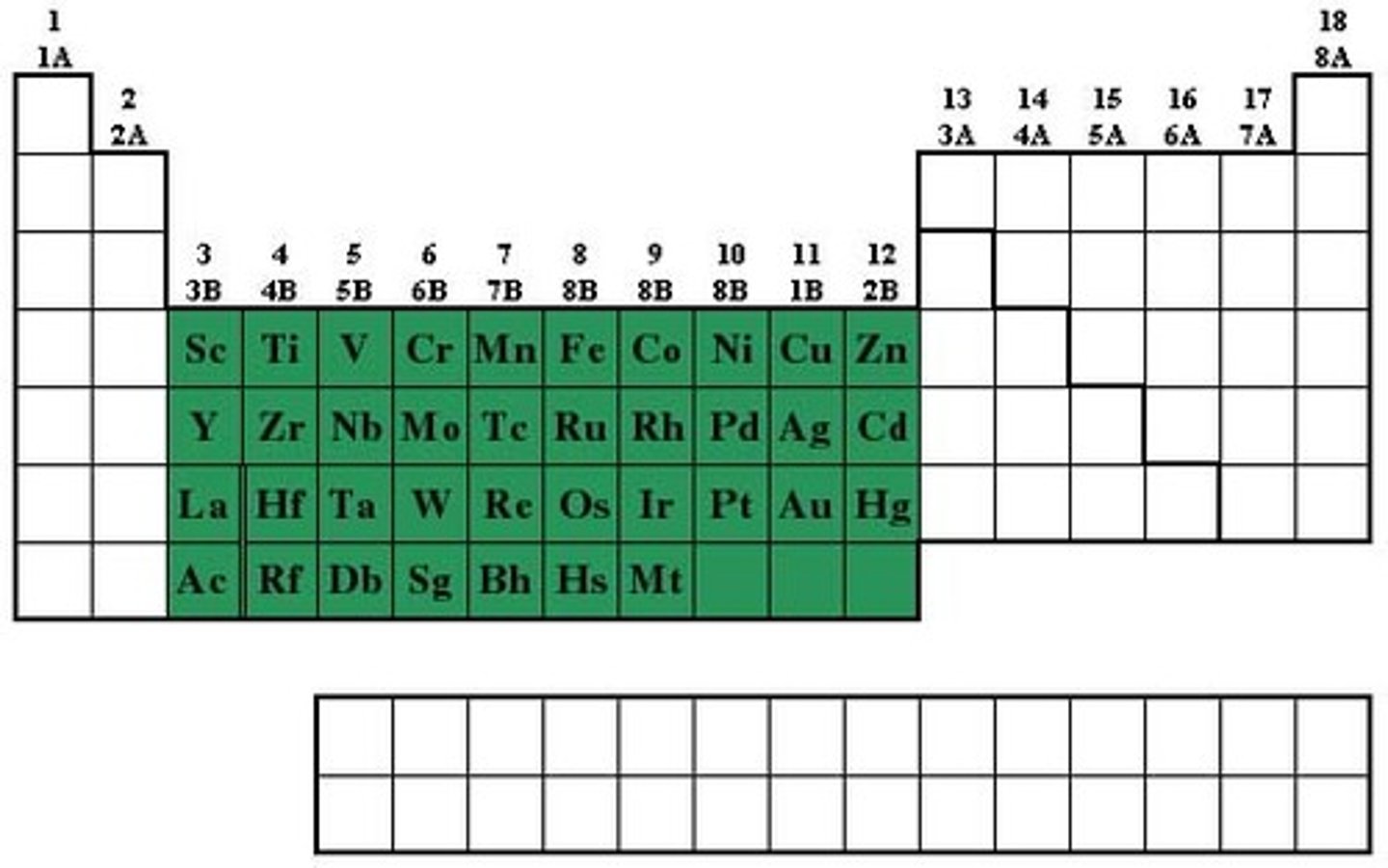
Halogens
Group 17, Contains nonmetals, 7 valence electrons in it's outermost energy level. Very reactive
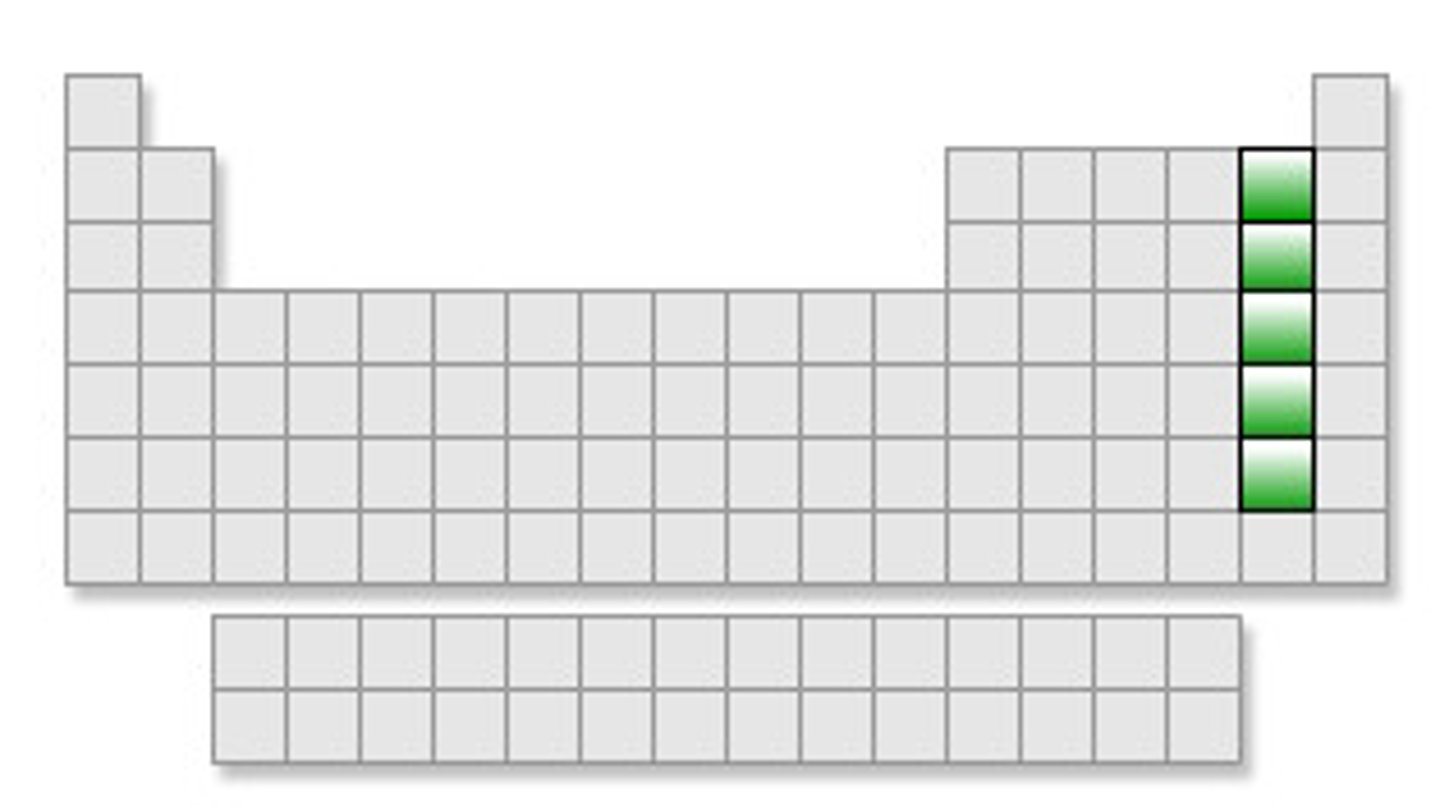
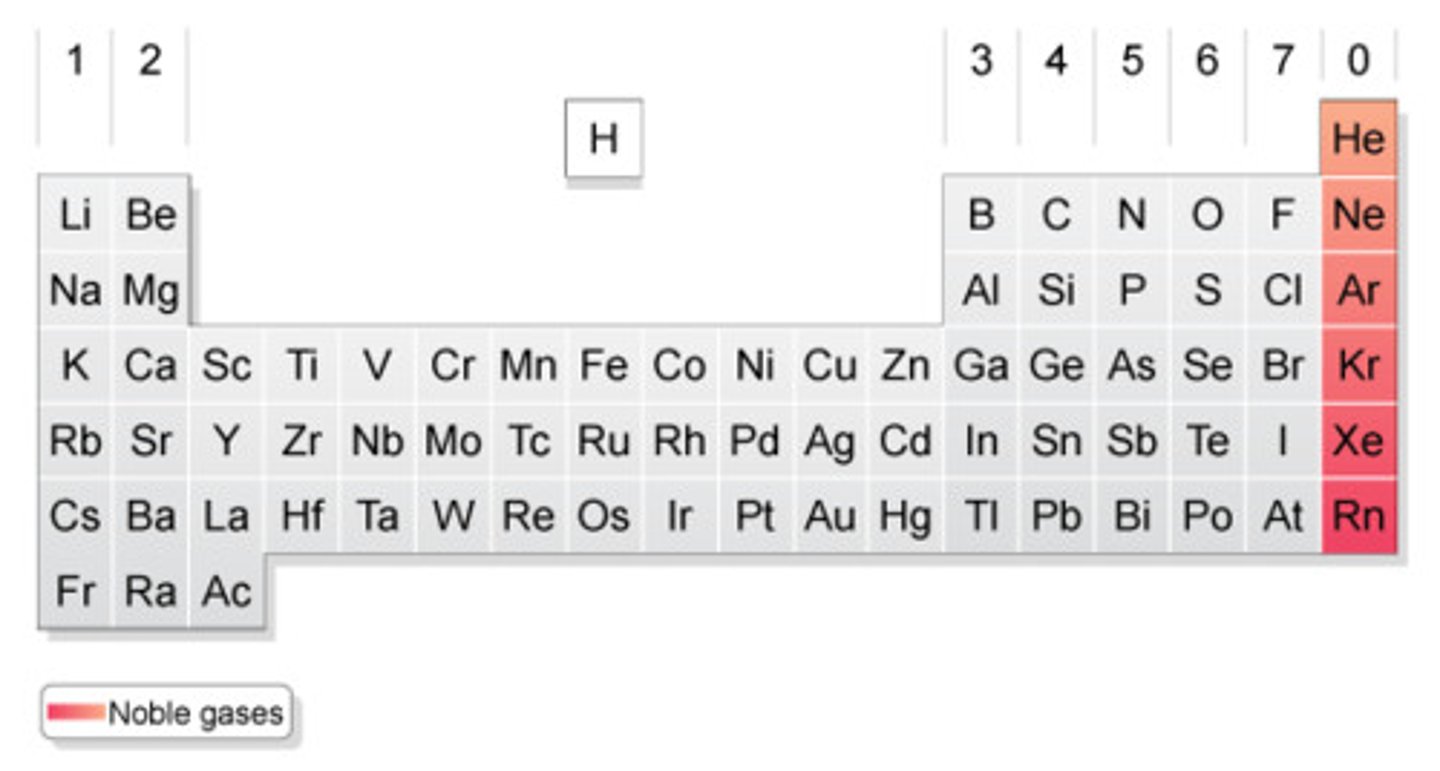
Noble gases
Group 18
Non-metals
Low conductivity, not ductile, not malleable, brittle, dull, gas at room temp
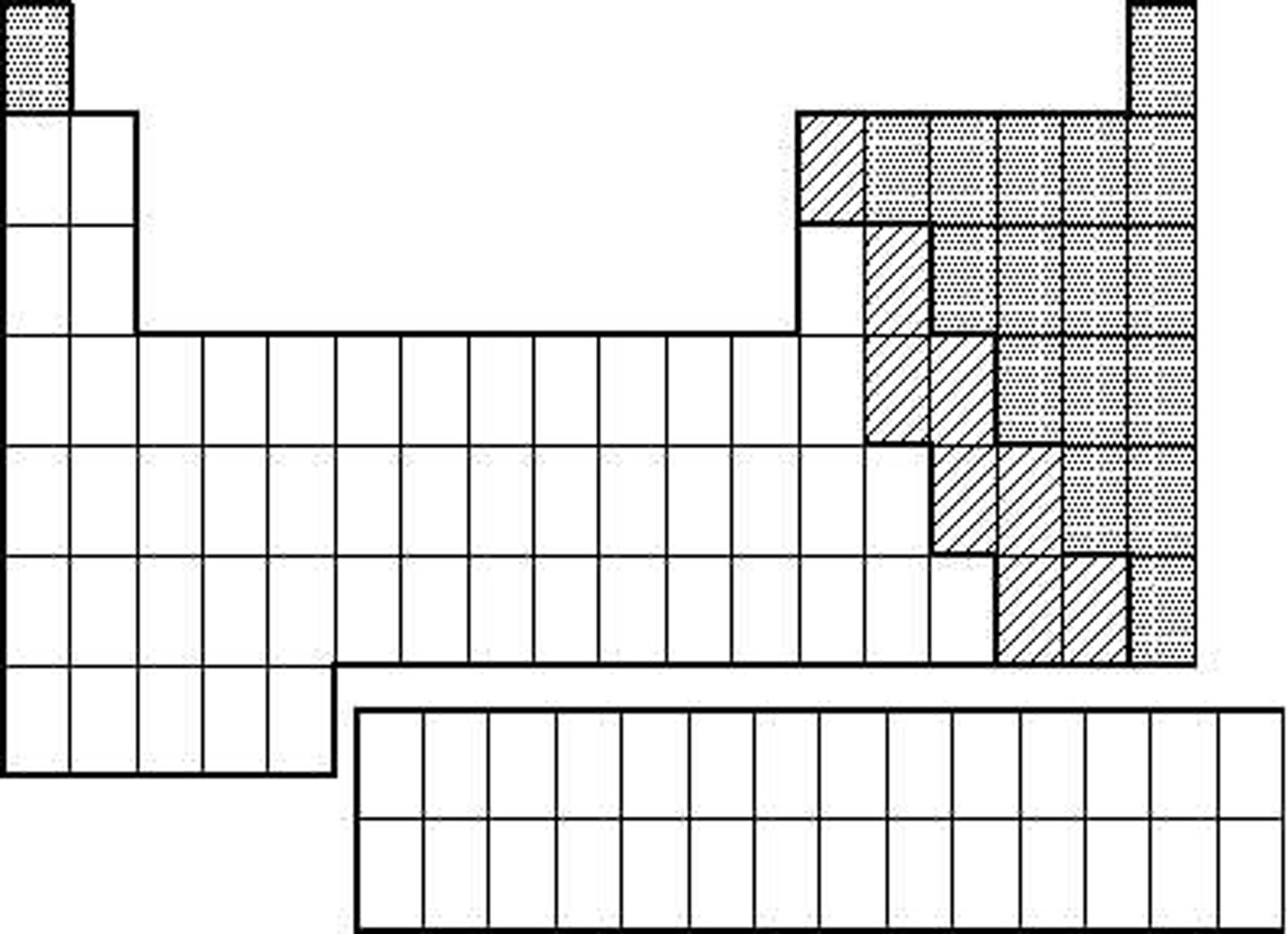
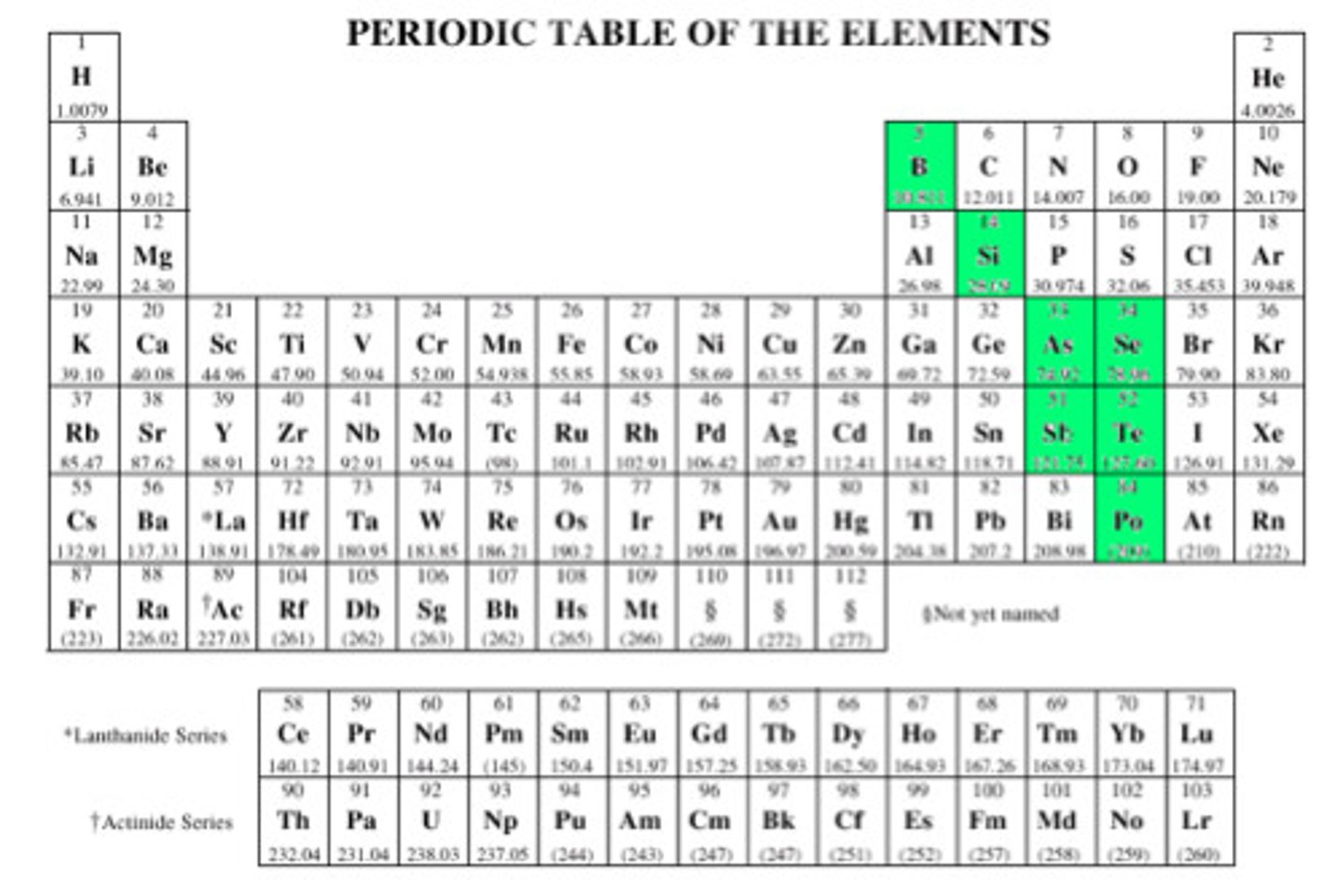
Metalloids
elements with properties that fall between those of metals and nonmetals
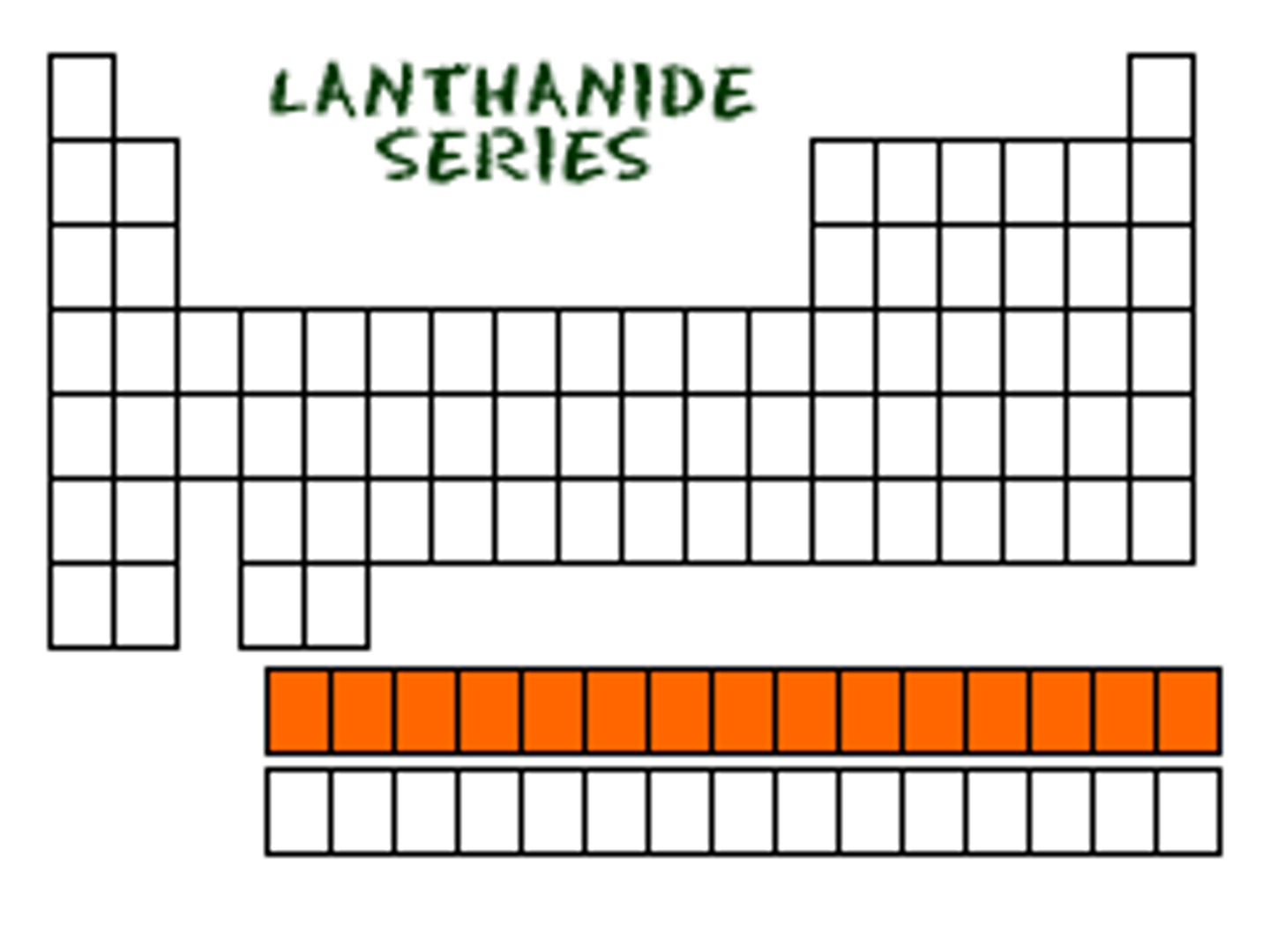
Lanthanoids
Elements 57-71
Actinoids
Elements 89-103
1
mono
2
di
3
tri
4
di
5
penta
6
hexa
7
hepta
8
octa
H
Hydrogen
He
Helium
Li
Lithium
Be
Beryllium
B
Boran
C
Carbon
O
Oxygen
F
Fluorine
Ne
Neon
Na
Sodium
Mg
Magnesium
Al
Aluminum
Si
Silicon
P
Phosphorus
S
Sulfur
Cl
Chlorine
Ar
Argon
K
Potassium
Ca
Calcium