StemUp: OCR A A level Biology 2.1.2 Biological Molecules
1/118
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
119 Terms
Describe the strength of hydrogen bonds (2)
- Individual hydrogen bonds are weak
- However, collectively together they are very strong
Where are hydrogen bonds commonly found? (4)
- Proteins
- Water
- DNA
- tRNA
How does hydrogen bonding occur between water molecules? (2)
- Water is a polar molecule due to uneven distribution of charge
- Bond forms between the delta positive hydrogen atom and the delta negative oxygen atom
What are the properties of water? (4)
- Important solvent in reactions
- A transport medium
- A coolant
- Provides a habitats for aquatic organisms
Describe how the properties of water make it suitable as a habitat for living organisms (4)
1. Water buffers temperature
2. So therefore, provides a stable internal environment
3. Cohesion between water molecules
4. Which enables small invertebrates to live on water surface
Describe how properties relating to the density of water contribute to the survival of organisms in a habitat (3)
- Ice is less dense than water so floats
- Floating ice on the surface of water acts as an insulator for organisms living in the water
- Ice will provide a habitat for some species
Describe how the properties of water make it suitable as a coolant (4)
- Water has a high specific heat capacity and a high latent heat of vaporisation
- This is due to the strong hydrogen bonds between the molecules
- Internal temperatures within plants and animals remain constant in external fluctuating temperatures
- As it provides a cooling effect in animals via sweating and a cooling effects in plants via the transpiration stream
Describe how the properties of water make it suitable as a transport medium (2)
- Cohesion between water molecules
- Forms a continuous column of water in the xylem of plants
Describe how the properties of water make it suitable as a solvent (3)
- Polar molecules dissolve readily in water
- Due to water being polar
- Which causes attraction to the solute molecules (the polar molecules)
What is one eukaryote and one prokaryote example of water acting as a solvent? (2)
- In bacteria, water acts as a solvent for nutrients and ions for biochemical reactions
- In human cells, water dissolves glucose and oxygen for respiration
What is one eukaryote and one prokaryote example of water acting as a transport medium? (2)
- In cyanobacteria, water helps transport dissolved gases like CO2 for photosynthesis.
- In plants, water transports nutrients and minerals through the xylem from roots to leaves.
What is one eukaryote and one prokaryote example of water acting as a coolant? (2)
- In thermophilic bacteria, water helps dissipate heat generated from metabolic reactions.
- In humans, sweating allows water to evaporate from the skin, cooling the body.
What is one eukaryote and one prokaryote example of water acting as a habitat? (2)
- Water serves as a habitat for aquatic bacteria, providing a stable environment for growth and reproduction
- Water bodies serve as habitats for fish and amphibians, supporting a wide range of life forms.
What is meant by monomers? (1)
Smaller units from which larger molecules are made
What is meant by polymers? (1)
Molecules that are made from a large number of repeating units joined together
What is a condensation reaction? (3)
- A chemical reaction that joins up two molecules
- With the formation of a chemical bond
- And the loss of a water molecule
What is a hydrolysis reaction? (2)
- A chemical reaction that breaks up the chemical bond between two molecules
- And the addition of a water molecule
What chemical elements do carbohydrates contain? (3)
- Carbon (C)
- Hydrogen (H)
- Oxygen (O)
What chemical elements do lipids contain? (3)
- Carbon (C)
- Hydrogen (H)
- Oxygen (O)
What chemical elements are found in all amino acids (and therefore all proteins)? (5)
- Carbon (C)
- Hydrogen (H)
- Oxygen (O)
- Nitrogen (N)
- Some also contain Sulphur (S)
What are the monomers of carbohydrates? (1)
Monosaccharides
What are three examples of monosaccharides? (3)
- Glucose
- Fructose
- Galactose
What type of monosaccharide is glucose? (2)
- Hexose sugar
- Which means it contains 6 carbon atoms
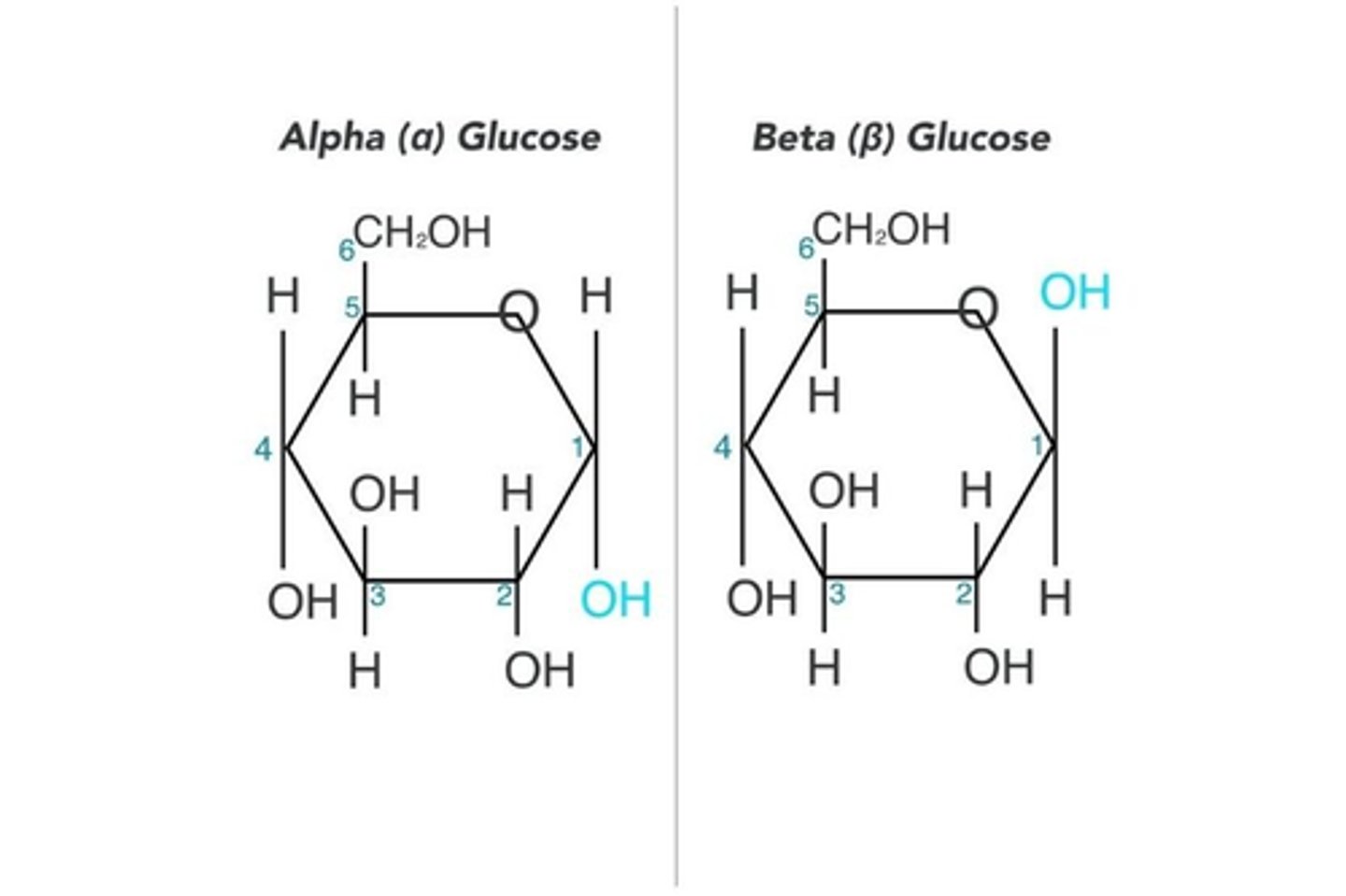
What are the two forms of glucose? (2)
- α-glucose
- β-glucose
How do you draw the two isomers of glucose? (2)
What type of monosaccharide is ribose? (2)
- Pentose sugar
- Because it has 5 carbons
How is the structure of glucose related to its function as the main energy source in animals and plants? (2)
- It is soluble, so it can be easily transported
- Its chemical bonds contain a lot of energy.
What type of bond joins monosaccharides together? (1)
Glycosidic bonds
How is a disaccharide formed? (3)
- When two monosaccharides are joined together
- By a glycosidic bond
- During a condensation reaction
How is a polysaccharide formed? (3)
- When more than two monosaccharides are joined together
- By a glycosidic bond
- During a condensation reaction
What is maltose made of? (2)
Alpha glucose + Alpha glucose
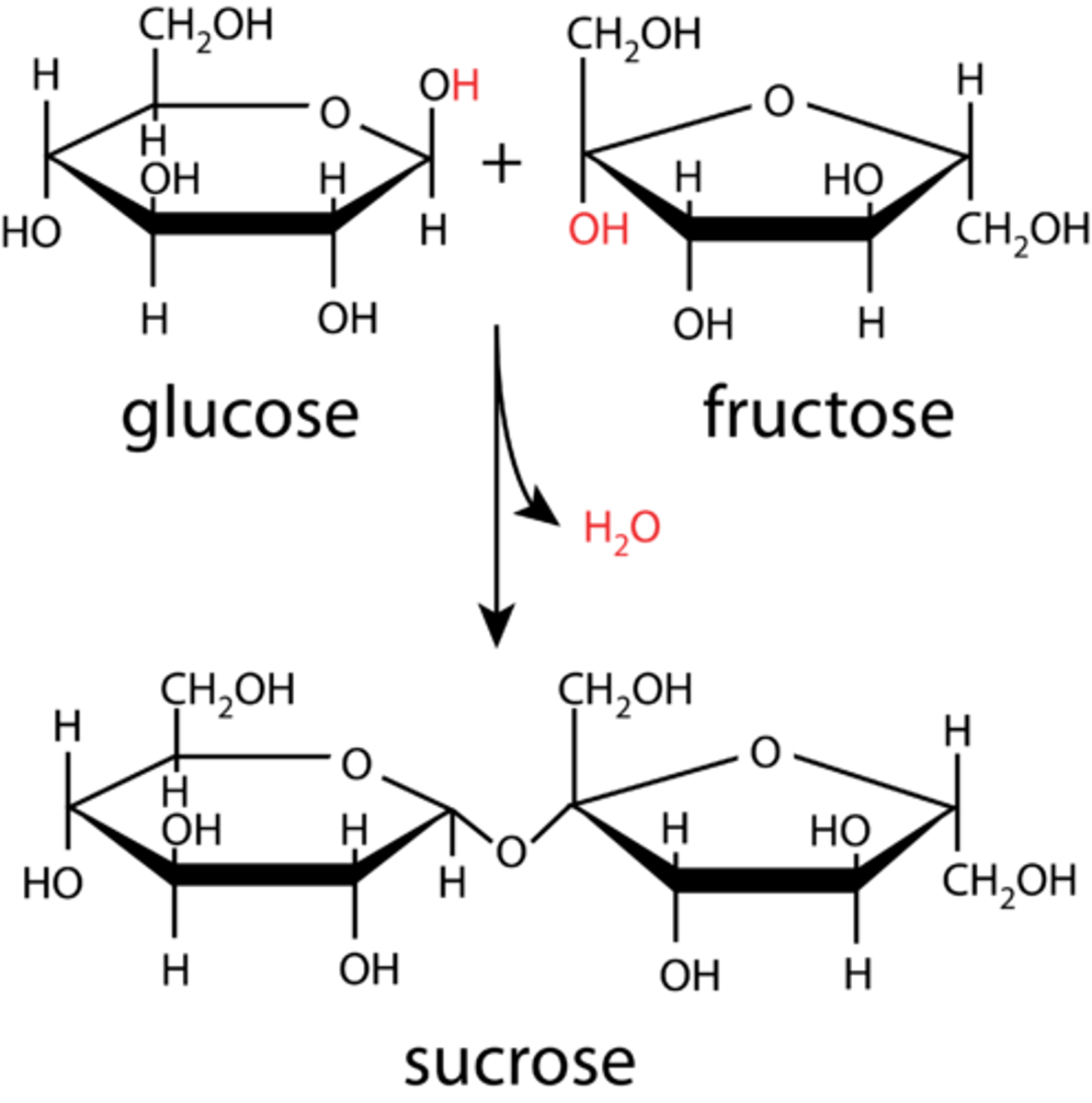
What is sucrose made of? (2)
α-glucose + fructose
What is lactose made of? (2)
α-glucose or β-glucose + galactose
Draw and label diagram of the condensation reaction that occurs to form sucrose (4)
What is the main energy store in plants? (2)
- Starch
- Excess glucose is stored as starch
Why is starch a good molecule for storage in plants? (3)
- Insoluble so osmotically inert
- Therefore, does not affect water potential
- So does not draw in water into plant cells
What are the two polysaccharides that make up starch? (2)
- Amylose
- Amylopectin
Describe the structure of amylose (3)
- Long unbranched chain of α-glucose molecules
- Joined by 1-4 glycosidic bonds
- Which give it a coiled structure so compact
Describe the structure of amylopectin? (3)
- Long branched chain of α-glucose molecules
- It has 1-4 glycosidic bonds and branches formed by 1-6 glycosidic bonds
- Resulting in a branched rather than helical structure
Why is the branched structure of amylopectin important? (2)
- Allows enzymes to easily access the glycosidic bonds
- Which enables quick release of glucose
What is the main energy store in animals? (1)
Glycogen
Describe the structure of glycogen (2)
- Highly branched chain of α-glucose molecules,
- Which allows enzymes to access the glycosidic bonds easily and hydrolyse glucose quickly
Why is glycogen a good storage molecule in animals? (3)
- Compact
- Has a high energy content for its mass
- Insoluble, so it does not affect the cell's water potential
What is the major component of cell walls in plants? (1)
Cellulose
Describe the structure of cellulose
- Made up of long, straight, unbranched chains
- Of β-glucose molecules
How are cellulose chains held together, and what function does this serve? (3)
- By hydrogen bonds to form microfibrils
- Which are strong fibers
- That provide structural support for plant cells
What is the structure of a triglyceride? (2)
- Consists of one molecule of glycerol
- With three fatty acids attached to it
What is the structure of a phospholipid? (2)
- Consists of one molecule of glycerol
- With two fatty acids and one phosphate group attached to it
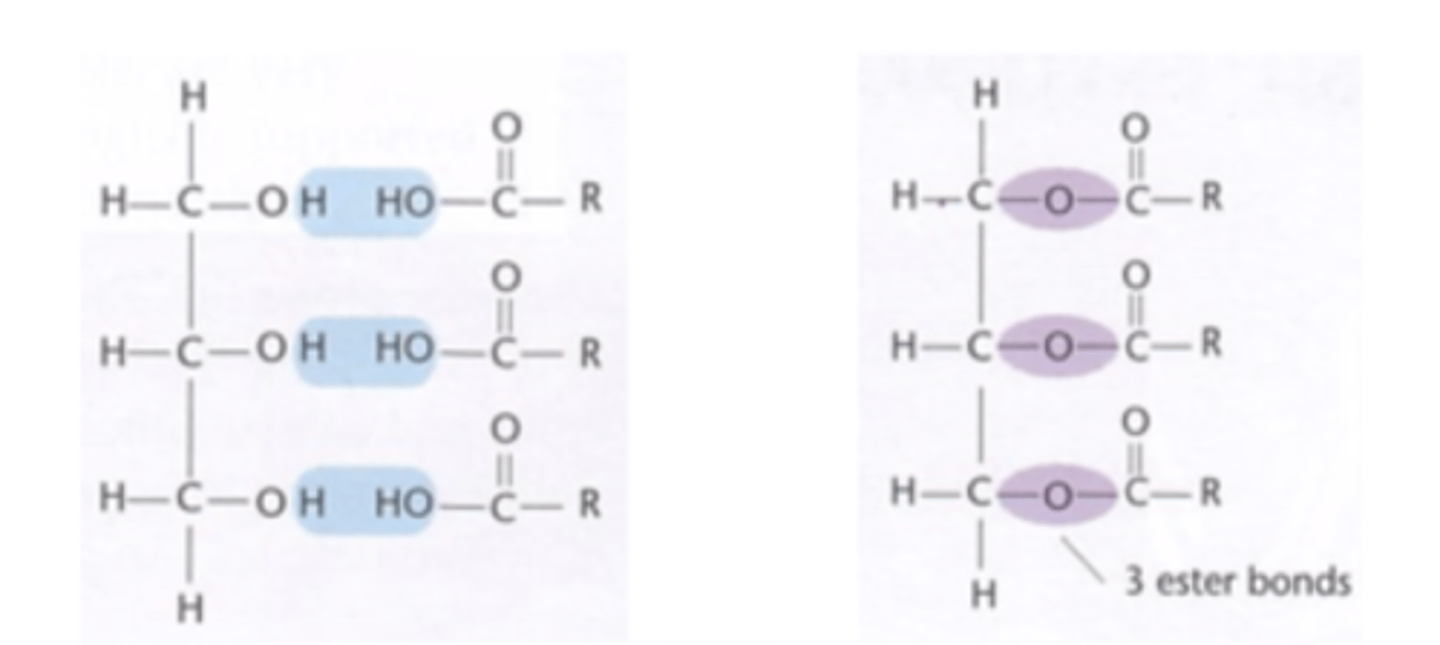
How are triglycerides synthesised? (2)
- By the formation of an ester bond
- Between each fatty acid and the glycerol molecule
How is each ester bond in a triglyceride formed? (3)
- Formed by a condensation reaction,
- Where one molecule of water is released.
- To form one triglyceride, three condensation reactions take place, releasing three molecules of water
Draw a diagram to show the condensation reactions that occur to form one triglycerides (6)
What is esterification? (1)
Process by which triglycerides are synthesised
How can triglycerides be broken down? (2)
- Hydrolysis of each ester bond
- To form glycerol and fatty acids
What is the difference between saturated and unsaturated fatty acids? (2)
- Saturated fatty acids do not have any double bonds between their carbon atoms
- Unsaturated fatty acids have at least one double bond between their carbon atoms
What is the primary function of triglycerides in plants and animals? (2)
- Function as energy storage molecules in plants and animals
- Some bacteria, such as Mycobacterium tuberculosis, also use triglycerides to store energy and carbon
Why are triglycerides good storage molecules? (3)
- Long hydrocarbon tails of the fatty acids contain a lot of chemical energy
- Which is released when they are broken down
- This is why lipids provide twice as much energy per gram as carbohydrates
Why do triglycerides not cause water to enter the cell by osmosis? (3)
- Triglycerides are insoluble
- So they do not cause water to enter cells by osmosis
- Preventing cell swelling
How do triglycerides form insoluble droplets in cells? (2)
- The fatty acid tails are hydrophobic
- These tails face inward, shielding themselves from water with their glycerol heads
Where are phospholipids found? (1)
In the cell membranes of all eukaryotes and prokaryotes
What structure do phospholipids form in cell membranes? (1)
The phospholipid bilayer
How do phospholipids arrange themselves in the phospholipid bilayer? (3)
- They have hydrophilic phosphate heads and hydrophobic fatty acid tails
- So they form a double layer
- With their heads facing outwards towards the water on either side of the membrane
Why is the centre of the phospholipid bilayer important for cell function? (3)
- Its hydrophobic
- Preventing water soluble substances from passing through it
- Making the membrane a barrier that controls what enters and leaves the cell
Describe the basic structure of cholesterol (2)
- Has a hydrocarbon ring structure attached to a hydrocarbon tail
- The ring structure has a polar OH group attached to it
What is the role of cholesterol in eukaryotic cell membranes? (2)
- Strengthens the cell membrane
- By interacting with the phospholipid bilayer
How does cholesterol fit into the phospholipid bilayer? (2)
- It is small and flattened
- So it can fit between the phospholipid molecules in the membrane
How does cholesterol interact with phospholipids to regulate membrane fluidity? (3)
- OH group interacts with phospholipid heads
- Hydrocarbon tail embeds alongside fatty acid tails of phospholipids
- Phospholipids pack closer together, making the membrane less fluid and more rigid
Draw the general structure of an amino acid (4)
- A central carbon with a hydrogen
- An amine group, a carboxyl group and an R group (variable group - the R group is the difference between different amino acids)
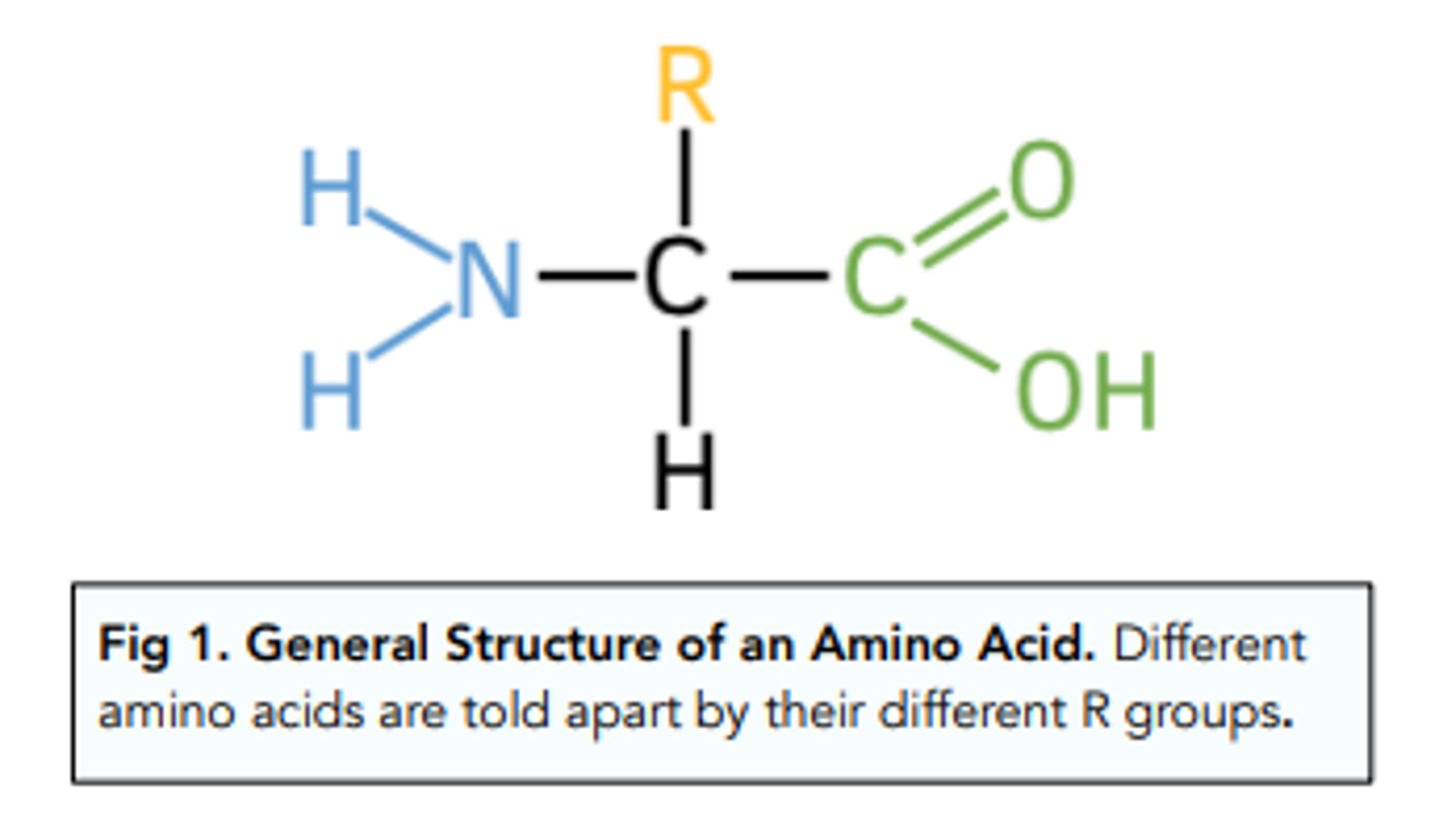
What are amino acids in relation to proteins? (1)
Monomers of proteins / polypeptides
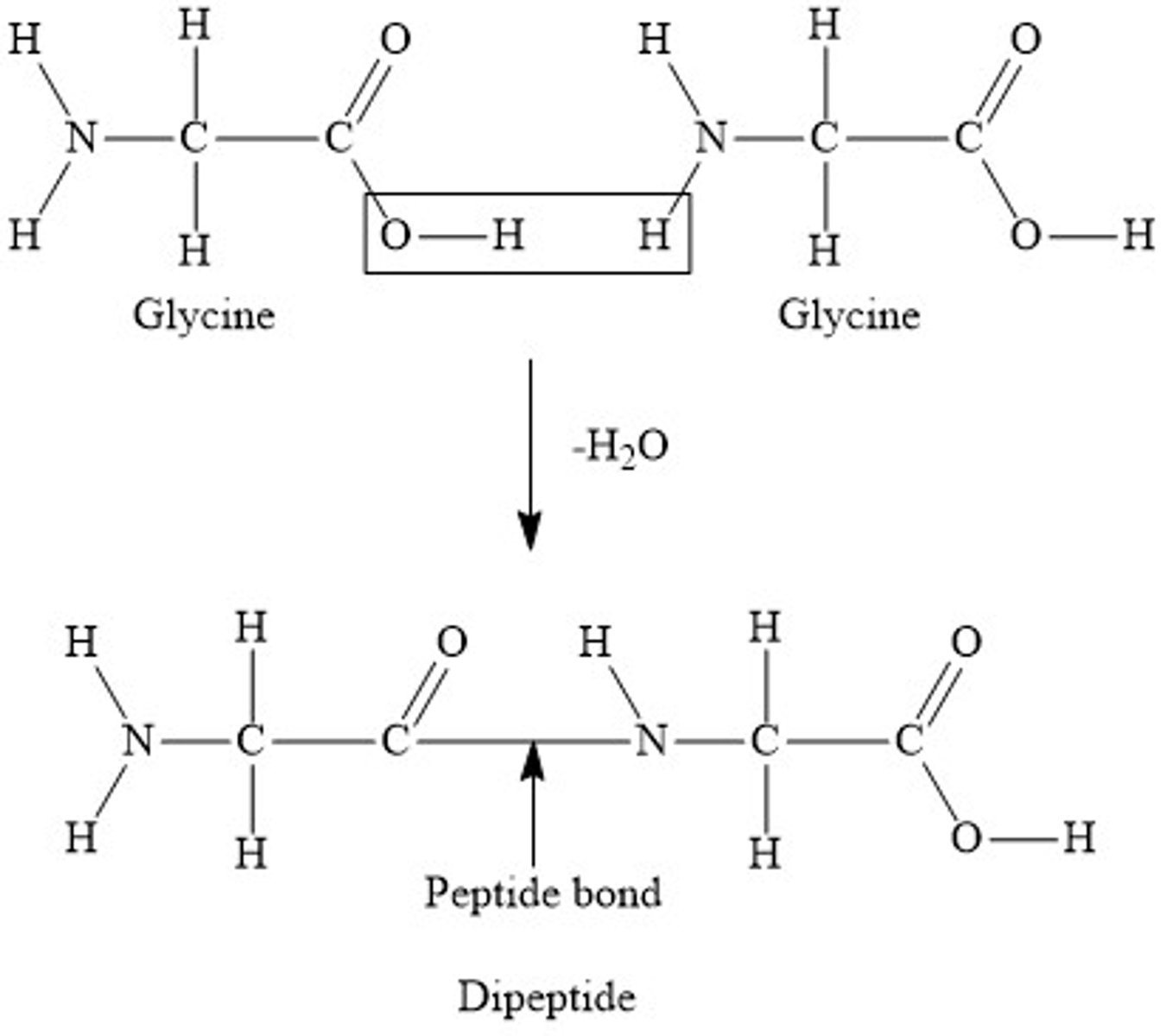
What is a dipeptide? (1)
- Formed when two amino acids are joined together
- By a peptide bond
What is a polypeptide? (2)
- Formed when two or more amino acids are joined together
- By a peptide bond
What are proteins made of? (1)
One or more polypeptides
How are amino acids joined together in proteins? (2)
- By peptide bonds.
- These bonds are formed by condensation reactions between the amino group of one amino acid and the carboxyl group of another
How can peptide bonds be broken? (2)
- By a hydrolysis reaction
- Which adds a molecule of water to break the bond
Draw and label a diagram of the formation and hydrolysis of a dipeptide (6)
NOTE: You do not have to learn any amino acid structure, you will be given this in the exam
What is the primary structure of a protein? (2)
- The sequence of amino acids in the polypeptide chain
- That are held together by peptide bonds
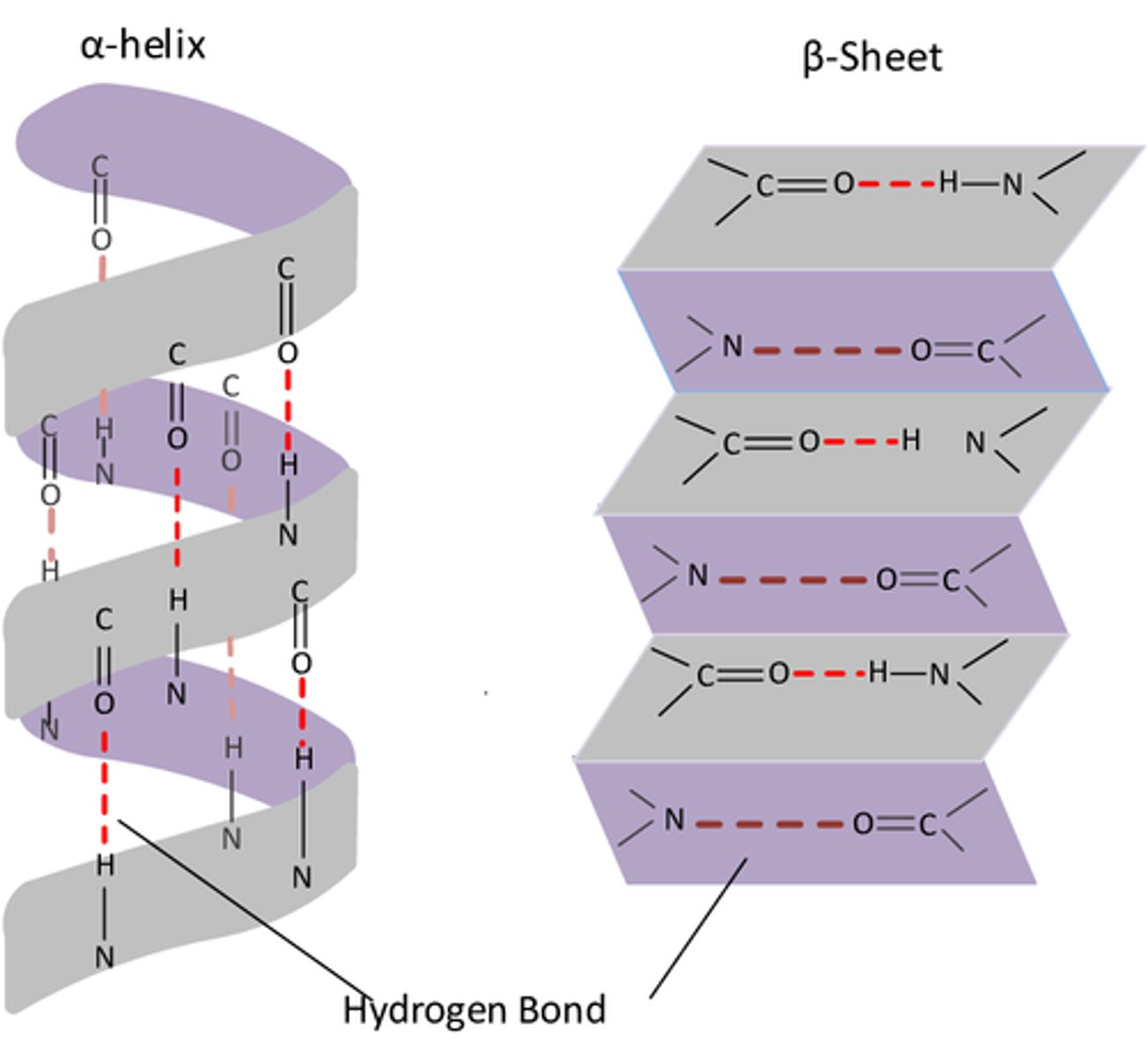
What is the secondary structure of a protein? (2)
- The coiling or folding of a polypeptide chain
- By hydrogen or ionic bonds
What are the two examples of secondary structures of a protein? (2)
- α-helix
- β-pleated sheet
What is the tertiary structure of a protein? (2)
- The further folding and coiling of the secondary structure to form the final 3D shape
- Due to hydrogen, ionic and sometimes disulphide bonds
What is the quaternary structure of a protein? (2)
- Proteins made up of more than one polypeptide chain
- E.g. haemglobin
What are globular proteins? (1)
Spherical and compact proteins
What are the properties of globular proteins? (2)
- Soluble
- Easily transported in fluids
What is haemoglobin, and what is its function? (3)
- A globular protein that carries oxygen around the body in red blood cells
- Contains 4 haem groups
- Consists of four polypeptide chains, each with a haem group
What is insulin, and what is its function? (2)
- Hormone that is secreted by the pancreas that helps regulate blood glucose levels
- Consists of two polypeptide chains held together by disulphide bonds
What is amylase, and what is its function? (3)
- Amylase is an enzyme that catalyses the breakdown of starch
- It consists of a single polypeptide chain
- Its secondary structure contains both α-helices and β-pleated sheets
What are the key properties of fibrous proteins? (4)
- Insoluble
- Strong
- Structural
- Fairly unreactive
What are three examples of fibrous proteins? (3)
- Collagen
- Keratin
- Elastin
What is collagen, and what is its function? (2)
- Collagen is a fibrous protein found in animal connective tissues (bone, skin, muscle)
- It is very strong, and minerals like calcium can bind to it to increase its rigidity
What is elastin, and what is its function? (2)
- Elastin is a fibrous protein found in elastic connective tissues
- It is elastic, allowing tissues to return to their original shape after being stretched
What is the role of the inorganic ion nitrate? (2)
- Absorbed from soil and is an important source of nitrogen
- Which is used to make amino acids and nucleic acids
What is the role of the inorganic ion hydrogen-carbonate? (2)
- Acts as a buffer
- To help maintain blood pH
What is the role of the inorganic ion chloride? (2)
- Involved in chloride shift, which helps maintain the pH of blood during gas exchange
- Also acts as a cofactor for amylase and is involved in some nerve impulses
What is the role of the inorganic ion phosphate? (2)
- Involved in photosynthesis and respiration
- Needed for the synthesis of many biological molecules, such as nucleotides (including ATP), phospholipids, and calcium phosphate (which strengthens bones)
What is the role of the inorganic ion hydroxide? (2)
- Affects the pH of substances
- More hydroxide ions than hydrogen ions present in a solution creates an alkali
What is the role of calcium, Ca²⁺? (4)
- Transmission of nerve impulses
- Release of insulin from the pancreas
- Cofactor for many enzymes (e.g., involved in blood clotting)
- Important in bone formation
What is the role of sodium, Na⁺? (2)
- Generating nerve impulses
- Muscle contraction
- Regulation of fluid balance
What is the role of potassium, K⁺? (4)
- Generating nerve impulses
- Muscle contraction
- Regulation of fluid balance
- Activates essential enzymes needed for photosynthesis
What is the role of hydrogen, H⁺? (2)
- Affects pH of substances
- Important for photosynthesis reactions in thylakoid membranes inside chloroplasts
What is the role of ammonium, NH₄⁺? (2)
- Absorbed from the soil by plants
- Important source of nitrogen used to make amino acids and nucleic acids
How would you carry out the biuret test for proteins? (4)
1. Add sodium hydroxide to make the solution alkaline
2. Add copper (II) sulfate solution
3. Protein present: solution turns purple
4. No protein: solution stays blue
How would you carry out the benedict's test for reducing sugars? (4)
1. Add Benedict's reagent to the sample
2. Heat in a water bath
3. Reducing sugar present: coloured precipitate forms (green to brick red)
4. No reducing sugar: stays blue