Carbonyl Chemistry
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
27 Terms
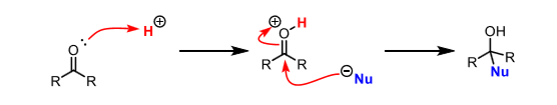
How to amplify electrophilicity of carbonyl group
Protonate the C=O before adding the nucleophile
Do aldehydes and ketones react via the same mechanism
Yes
Are aldehydes or ketones more reactive?
Aldehydes as the alkyl groups in ketones are much bulkier than the H atoms and alkyl groups also weak electron donors
Aldehyde/Ketone + Water
Diol
Alcohols + aldehydes/ketones
Hemiacetals
During acetal formation, to favour acetal formation…
add excess of diol/alcohol or remove water
During acetal formation, to favour aldehyde/ketone formation (reverse reaction)…
add excess water
Aldehyde/Ketone + Amine
Imine + water
How to test for a methyl adjacent carbonyl?
Iodoform test:
Add I2 in NaOH solution
Positive result forms a carboxylic acid and iodoform - yellow precipitate (CHI3)
How to distinguish between an aldehyde and ketone?
Fehlings test:
Add Cu2+ (Fehling’s A) in sodium tartrate & NaOH solution (Fehling’s B)
Positive result reduced Cu2+ to Cu+ and the aldehyde is oxidised to a carboxylic acid - froms a red precipitate
How to test for a specific halide group
Silver Nitrate test:
Add AgNO3
The Ag+ ions react with the halide to from AgX
Precipitate forms: White for Cl, Cream for Br, Yellow for I
Why are acyl chlorides very reactive?
Cl is an electronegative substituent → strongly withdraws electron density
Cl is a larger atom → minimal interaction between Cl lone pairs and C=O (no resonance stabilisiation)
Cl is a good leaving group as it is very stable
Why are amides very unreactive?
NR2 is a moderately electronegative substituent → less electron withdrawing than other substituents
N atom is similar in size to C atom → strong interaction between N lone pairs and C=O (very strong resonance stabilisation)
What is a leaving group
A substituent that is good at accepting a negative charge
What conditions does acyl chloride hydrolysis react under?
Mild conditions, no heating, neutral pH
Why is the esterification of acyl chlorides irreversible?
Cl- is a poor nucleophile
Basic ester hydrolysis
Base, H2O, H+ (acid workup)
Acidic ester hydrolysis
H+, H2O
Why can amides be synthesised from amines + carboxylic acids
Amines are basic so react with a carboxylic acid to form an ammonium salt
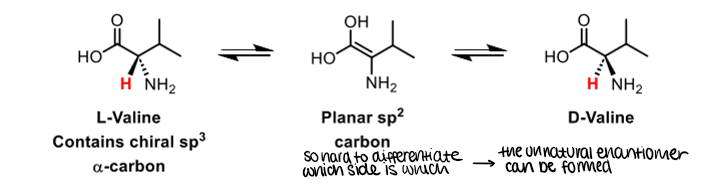
Tautomers
Isomers of the same compound which interconvert by migration of a H+ and a double bond
When can enolisation occur?
When there are protons alpha to the carbonyl
What are the consequences of enolisation
One issue is the racemisation of chiral centres next to a carbonyl group
What is an aldol
A molecule containing both a hydroxyl and carbonyl group
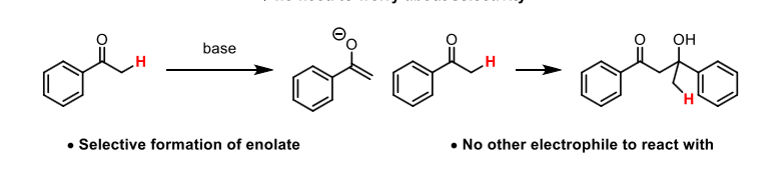
Self-Aldol reactions
The electrophile and nucleophile derive from the same carbonyl compound
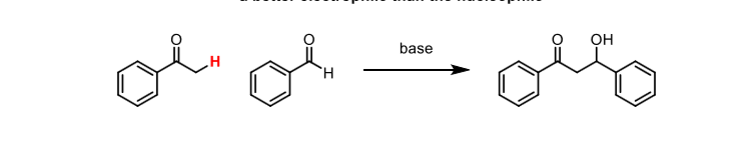
Cross-Aldol reactions
The electrophile should be unable to undergo enolisation and a better electrophile than the nucleophile
Why should asymmetrical ketones generally be avoided?
The are two potential enols/enolates that can be formed. To avoid this, use aldehydes or ketones where one side is non-enolisable.
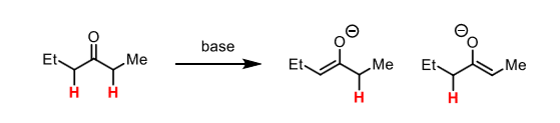
What is an enol?
A molecule containing a hydroxyl group bonded to a carbon involved in a C=C