Chapter 1 - states of matter and changes (intro?)
1/35
Earn XP
Description and Tags
um. We have this test on a Wednesday. Made this 1 day prior. You're joe-king. (gl) We can cook
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
36 Terms
Liquid → Gas
Vaporzation
What is vaporization?
Endothermal, speeds up
Gas → Liquid
Condensation
What is condensation?
Exothermic, slowing down
Gas → Solid
Deposition
What is deposition?
Exothermic, slowing down
Solid → Gas
Sublimation
What is sublimation?
Speeds up, Endothermic
Solid → Liquid
Melting
What is melting?
Speeding up, endothermic
Liquid → solid
freezing
What is freezing?
slowing down, exothermic
What is a vapor? (Explain)
When you have to apply heat, different temperature in room
What is a gas? (Explain)
Same as temperature in the room, you can’t see it
What is evaporization? (Explain)
happens naturally, doesn’t have heat applied
what is vaporization? (Explain)
happens controlled, has heat applied
what happens with water boiling?
tries to become gas, gravity pushes it down, particles are moving and colliding, atmosphere pressure pushes it down, vapor pressure becomes stronger causes particles to go up (Once vapor pressure=atmosphere pressure=boiling point)
What is the Kinetic Molecular Theory?
The movement of particles explination
3 examples in the molecular theory
particle motion, size, energy
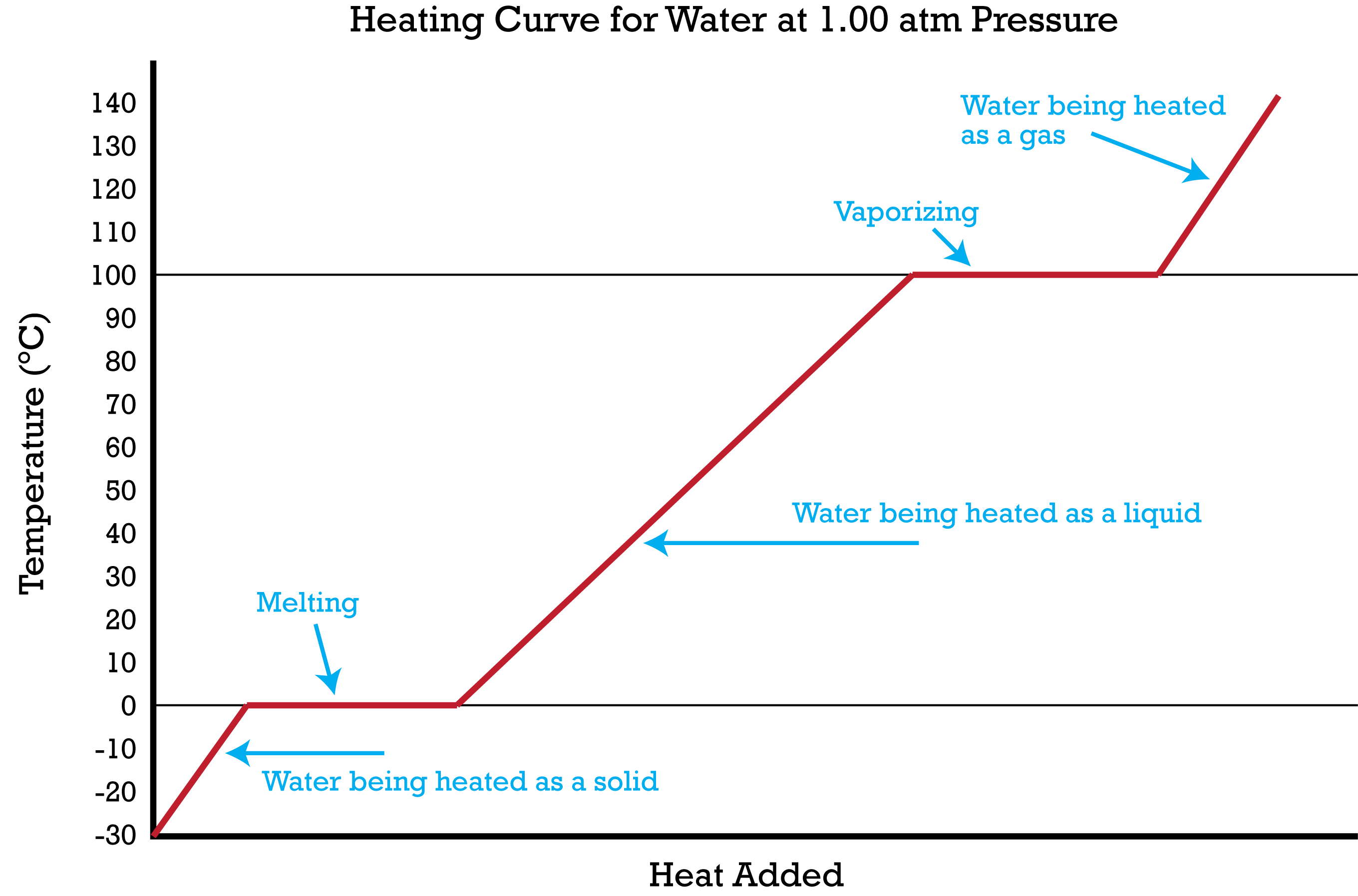
name of diagram?
Heating and cooling diagram
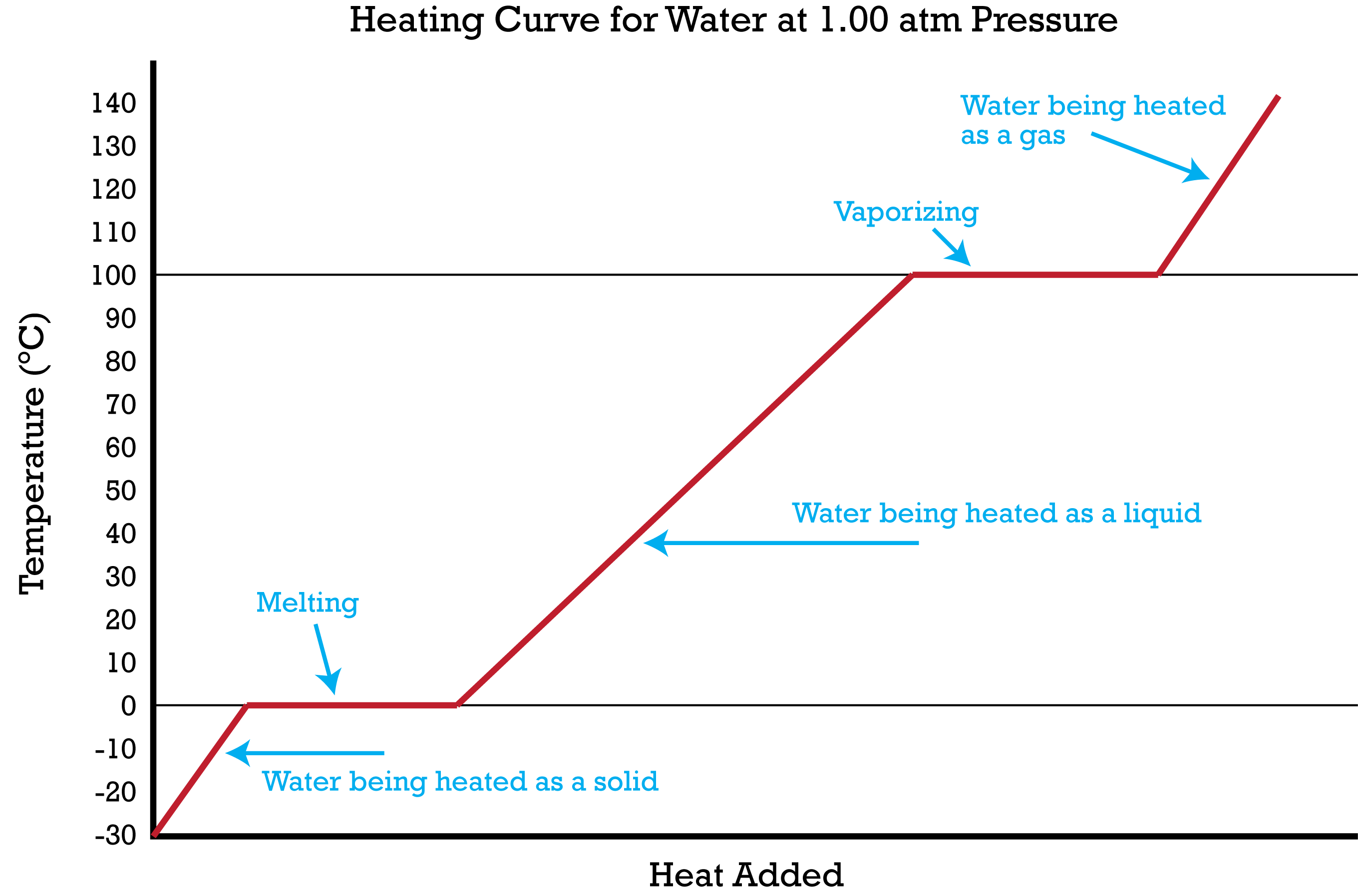
Where is the boiling point?
100c
Where is the melting point?
0c
STP should be what
ST 0c
SP 1atm
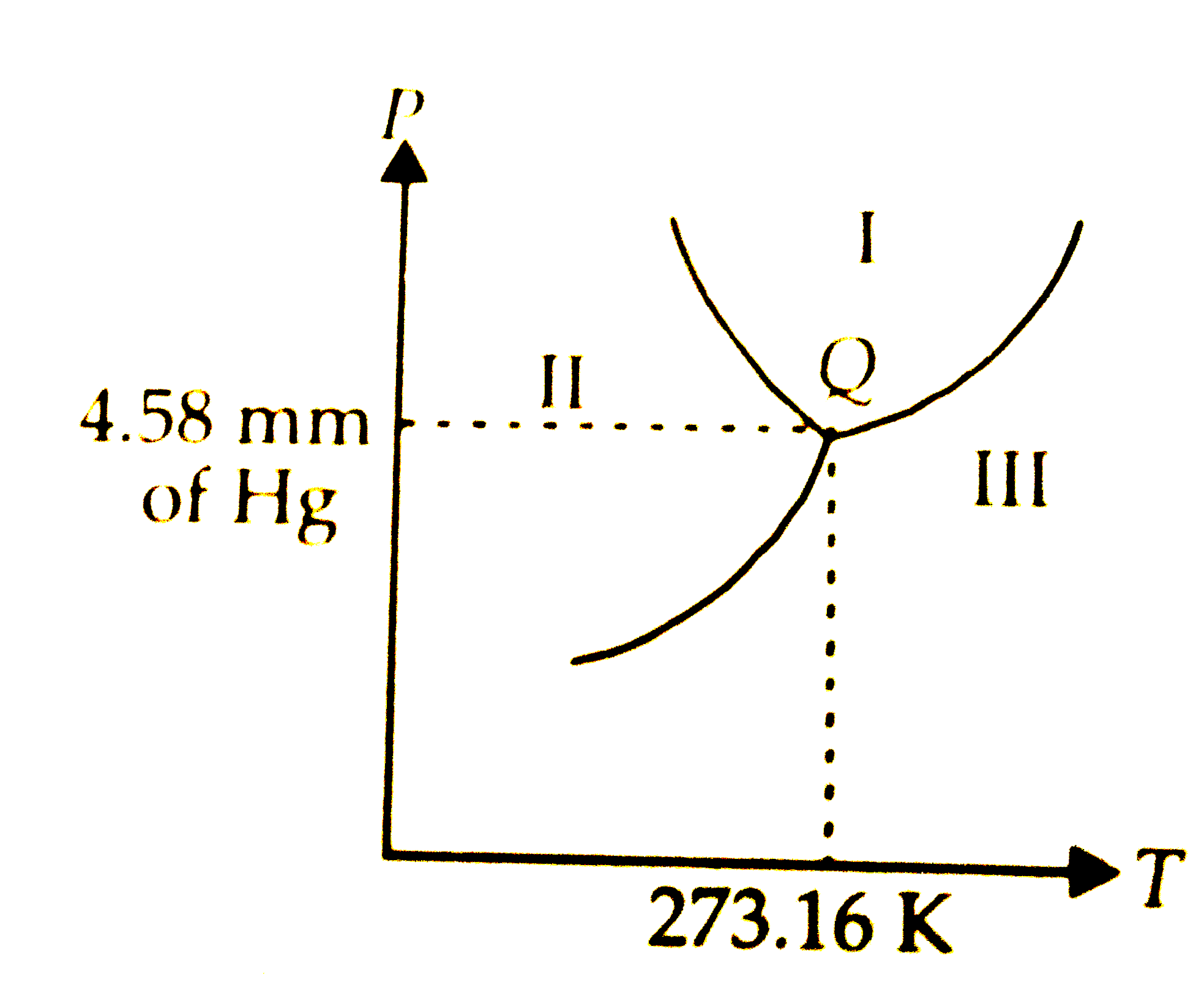
Tripple point
where all 3 points meet
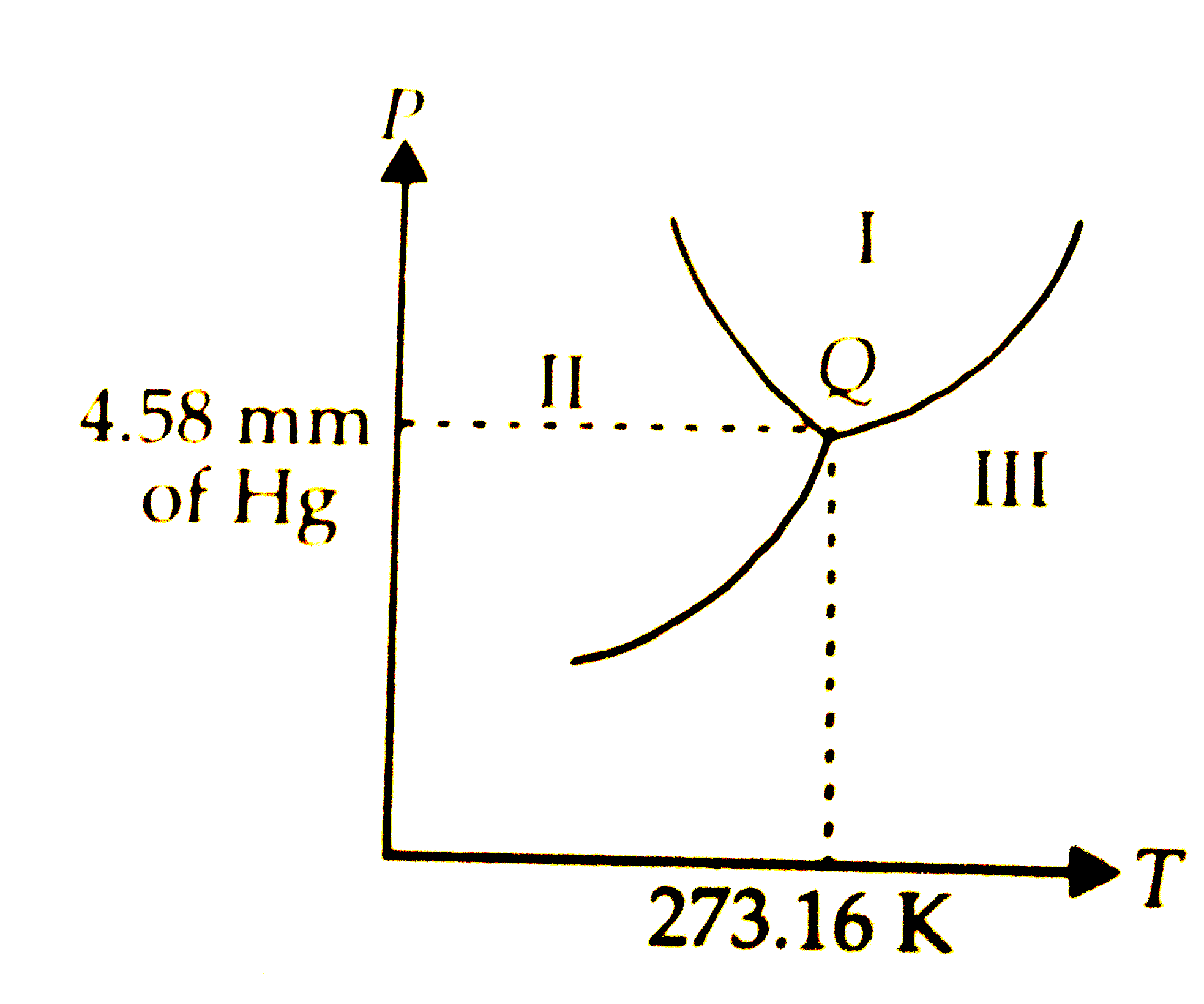
Where is gas?
iii
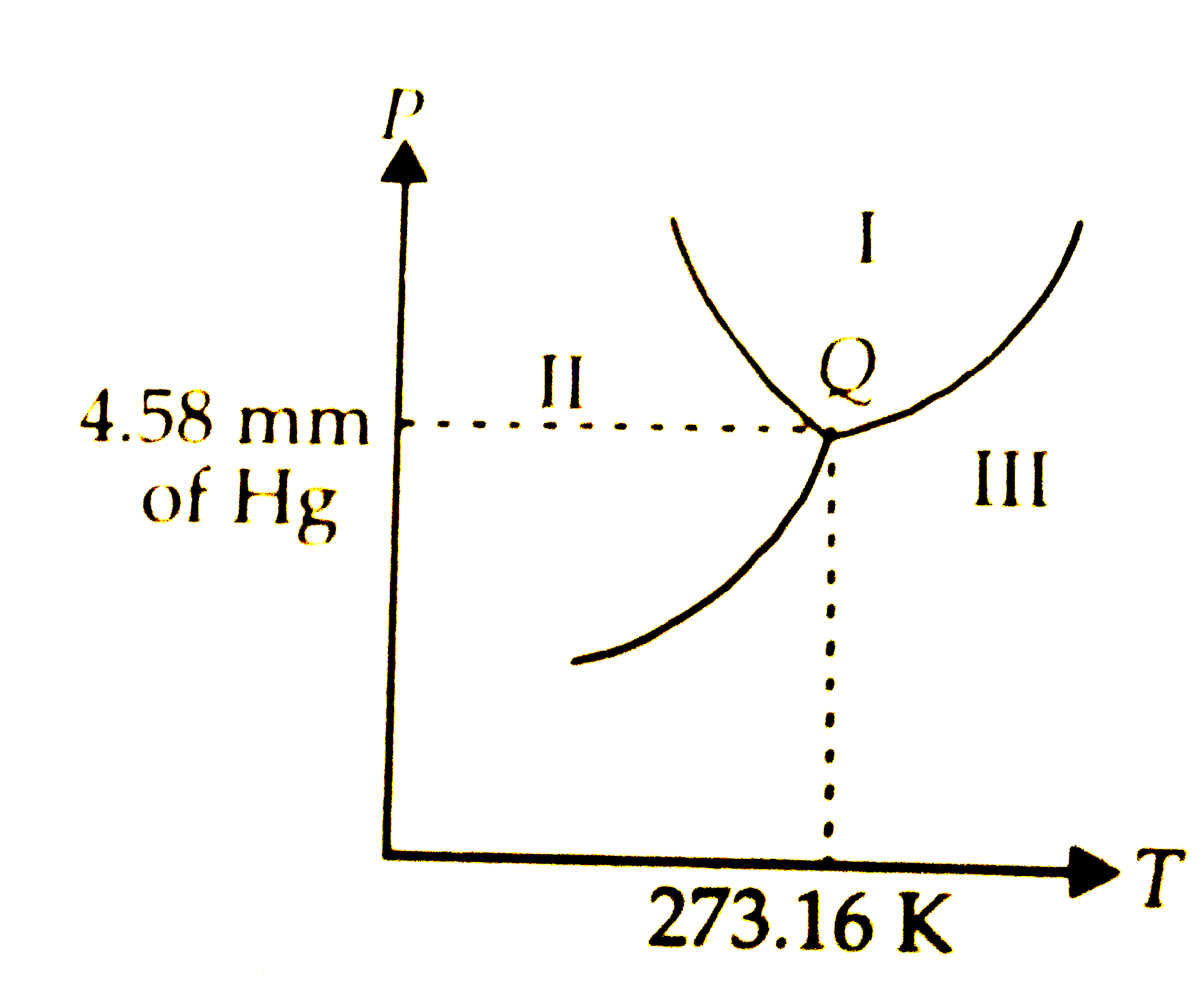
Where is liquid?
i
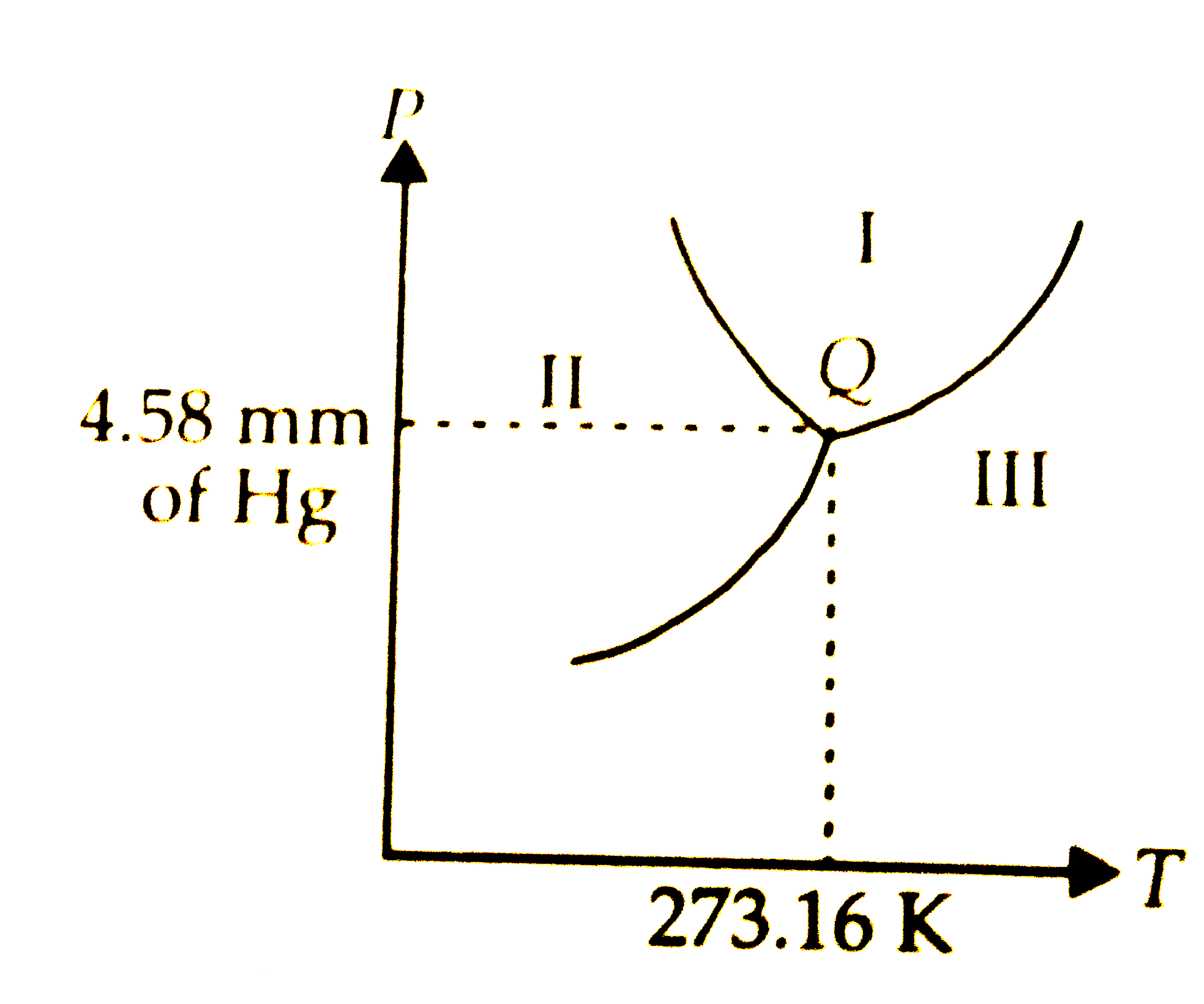
Where is solid?
ii
How to find boiling/freezing temperatures?
must hit the designated line
Critical point
critical temp and pressure is where water cannot coexist as a liquid (superficial state)
what is a superficial state?
substance behaving with properties both a liquid and a gas.
temp stays what during phase change?
the same
How does gas compression occur?
due to having a weak force they have no attraction to each other, being far apart and compressed.
How does a gas expand?
due to going in a straight constant random line until they collide with something, expanding everywhere.
Solid (What is this?)
defined volume and size, particles are still absolute, strong attraction
gas (what is this?)
undefined volume and size, separated by space, no attraction
liquid (what is this?)
indefined shape and volume, weaker attraction, higher energy