Chapter 6 Early Experiments in Quantum Theory - The Nature of Light and Matter
1/56
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
57 Terms
Meters to nanometers
m • (nm/(10^-9m)) = nm
Nanometers to Meters
nm • ((10^-9)/nm) = m
Pico-meters to Meters
pm • ((10^-12)/pm) = m
Meters to Picometers
m • (pm/(10^-12m)) = pm
Speed of Light in a Vacuum (c)
= 2.998•10^8 m/s
Formula for Speed of Light
c = 𝝀•ν = wavelength • frequency
When waves constructively interfere, what parameter changes in the resultant wave?
Intensity, changes as a result.
What is the relationship between wavelengths and frequency?
Wavelength and frequency are inversely proportional.
The Electromagnetic Spectrum (in order of increasing frequency)
1. Radio Waves
2. Microwaves
3. Infrared Radiation
4. Visible Light Spectrum
5. Ultra Violet Light
6. X-Rays
7. Gamma Rays
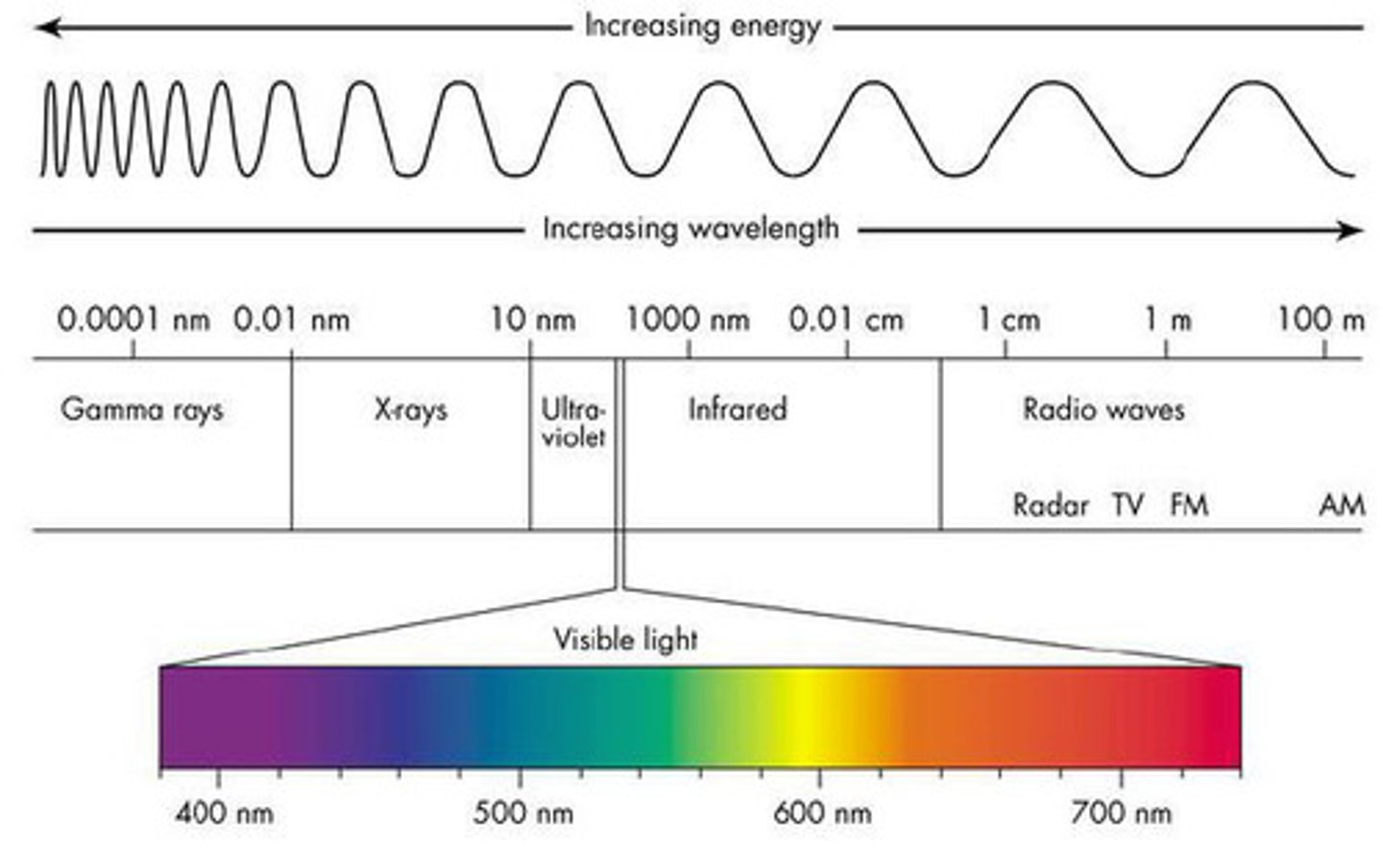
Radio Waves Frequency and Wavelength
Lowest Frequency; Frequency Range: 1 Hz - 10^9 Hz, Wavelength Range: 1 m - 10^9 m or 10^9 nm -10^18 nm
Radio Waves
Radio waves are able to interact with tiny magnetic fields generated from individual proton nuclei.
Microwaves Frequency and Wavelength
Frequency Range: 10^8 Hz - 10^11 Hz, Wavelength Range: 10^-3 m - 1 m or 10^6 nm - 10^9 nm
Microwaves
Interact with water molecules, thereby causing them to rotate and vibrate substantially.
Infrared Radiation Frequency and Wavelength
Frequency Range: 10^11 Hz - 10^15 Hz,
Wavelength Range: 10^-6 m - 10^-3 m or 10^3 nm - 10^6 nm
Infrared Radiation
The energy associated with any warm object; it excites molecular vibrations.
Visible Region of the Spectrum Frequency and Wavelength
700 nm - 400 nm
Frequency Range: 10^15 Hz
Wavelength Range: 10^-7 m - 10^-6 m or 100 nm - 1,000 nm
Visible Region of the Spectrum
The part of the electromagnetic spectrum that humans can see, in order of increasing frequency: Red, Orange, Yellow, Green, Blue, Indigo, Violet (ROYGBIV).
Ultraviolet Light Frequency and Wavelength
Frequency Range: 10^15 Hz - 10^17 Hz,
Wavelength Range: 10^-8 m - 10^-7 m, 10 nm - 10^-2 nm
Ultraviolet Rays
Ultraviolet is defined as radiation with wavelengths shorter than visible light.
X-Rays Frequency and Wavelength
Frequency Range: 10^17 Hz - 10^19 Hz,
Wavelength Range: 10^-11 m -10^-8 m or 10^-2 nm - 10 nm
X-Rays
Electromagnetic waves with wavelengths just shorter than those of ultraviolet rays
Gamma Rays Frequency and Wavelength
Frequency Range: 10^19 Hz - 10^24 Hz,
Wavelength Range: 10^-15 m -10^-11 m or 10^-6 nm - 10^-2 nm
Gamma Rays
A photon of electromagnetic radiation of very short wavelength, less than about 0.01 nanometer, and very high energy, greater than about 100,000 electron volts. Gamma rays are emitted in the decay of certain radioactive nuclei and in electron-positron annihilation.
Photoemission Spectrum
When the vapor of an element absorbs incident energy it gives off a unique color, the emitted light is passed through a prism, which can then be used to identify the element, by the colors that are now visible; These vertical lines are called the ______________.
Photoemission Spectroscopy
An experimental method used to determine the electronic structure of atoms and molecules.
Rydberg Equation
(1/𝝀) = R • [(1/nf^2) - (1/ni^2)], where R = 1.097•10^7m^-1, and nf< ni
![<p>(1/𝝀) = R • [(1/nf^2) - (1/ni^2)], where R = 1.097•10^7m^-1, and nf< ni</p>](https://knowt-user-attachments.s3.amazonaws.com/09ddcb5b-3051-45fb-b81d-3d7c757103ff.image/jpeg)
Rydberg Constant (R)
R= 1.097•10^7m^-1
or
R = 3.29 • 10^15 Hz
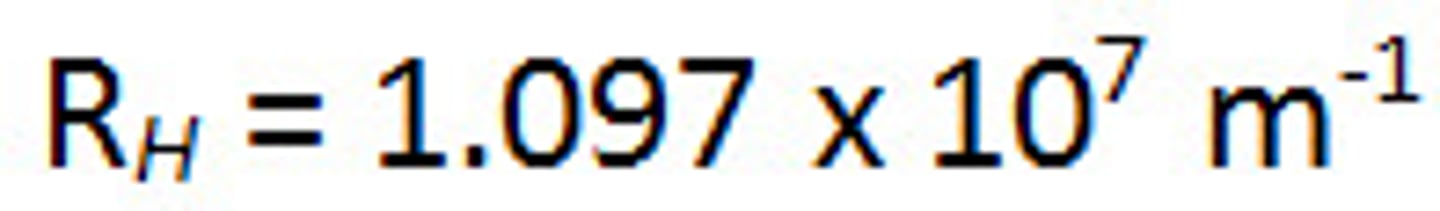
As nf increases while holding ni constant, what happens to λ?
𝝀 increases
Rydberg Equation Expressed in Hz
ν = R [(1/nf^2) - (1/ni^2)], where R = 3.29 • 10^15 Hz
As nf increases while holding ni constant, what happens to ν ?
ν decreases
What is the apparent relationship between energy and frequency?
Directly Proportional
Rayleigh-Jeans Law
Energy Density = constant • T • ν^2
What does the Rayleigh-Jeans Law imply?
That as temperature increases so does energy density, and that as frequency increases infinitely energy density increases infinitely.
Quanta
The bundle of electromagnetic energy that is absorbed or emitted by matter.
Photons
A quantum, or discrete quantity, of light energy that behaves as if it were a particle.
Planck's Equation
E = hv, where h=6.626•10^-34 J•s
Energy Given Speed of Light
E = h •(c/𝝀)
Wavelength Given Energy
𝝀 = h• (c/E)
Threshold Frequency
The minimum frequency required for metal electrons to undergo the photoelectric effect.
As more energy impacts the metal surface, electrons are ejected at which of the following?
A higher Kinetic Energy.
Photoelectric Effect
KE = (1/2) • m•v^2 = h•ν - 𝜙
What variable from the Rydberg equation also appears in Bohr's Radii?
The variable is n.
Which constant appears in both equations?
h
Bohr Radius
When n=1, r=5.29•10^-11
The Bohr radius theoretically is the __________ distance an electron can approach the nucleus in the hydrogen atom.
closest, smallest, nearest, shortest, and tiniest
What is Bohr's major contribution to quantum mechanics?
Bohr proposed that each orbit had an energy associated with it.
Bohr Frequency Condition
En = -R (1/n^2), where R = 2.18 • 10^-18 J, and n=1, 2, 3....
The less negative energy corresponds to an electron being __________ tightly held.
less
Energy of Emitted Photon
∆En = -R [(1/nf^2) - (1/ni^2)], R = 2.18 • 10^-18 J
De Broglie's Equation
𝝀 = (h/p) = (h/mv), where mass is in kg, velocity is in m/s, (planck's constant) h = 6.626•10^-34 m^2 kg/s, and p is linear momentum.
The number of full waves that span is __________ the value of n.
half
Standing Waves
L = n (𝝀/2), where n is a positive whole number.
If you wanted to use light to spot an electron's location, the electromagnetic wave you use (as in a microscope) would need to have a wavelength __________ than the electron's de Broglie wavelength.
shorter
Any wavelength longer than that of an electron would be too large for imaging. It follows that a short wavelength electromagnetic wave would have a __________ energy.
larger
Heisenberg's Uncertainty Principle
∆x∆p ≥ (h/4𝜋), where ∆p = m∆v = uncertainty in momentum in kg, ∆x = uncertainty in position (velocity in meters)
Notice that the right side of the equation is a constant > 0. Can either uncertainty be equal to zero?
No.
In order to maintain the constant of the right side of the equation, how are Δp and Δx related?
Inversely related