Topic 2: Electronegativity
0.0(0)
Card Sorting
1/3
There's no tags or description
Looks like no tags are added yet.
Last updated 1:37 PM on 5/29/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
4 Terms
1
New cards
Define electronegativity
Electronegativity- ability for an atom to attract electrons towards itself in a covalent bond
2
New cards
What type of bond do two molecules have when they have a difference of electronegativity of 0? (also draw the shared electrons on the bond, using Cl-Cl as the example)
Purely covalent
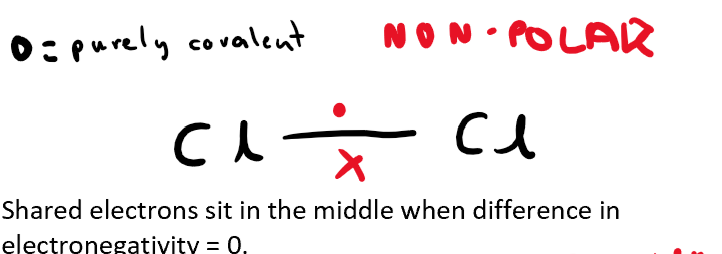
3
New cards
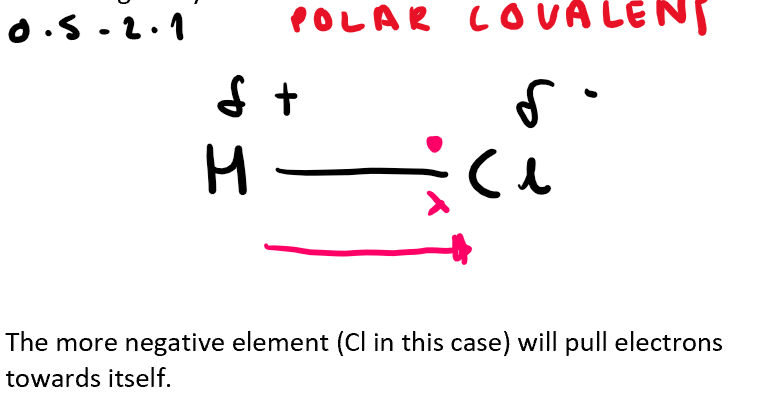
What type of bond do two molecules have when they have a difference of electronegativity of 0.5 - 2.1? (also draw the shared electrons on the bond, using H-Cl as the example)
Polar bond
4
New cards
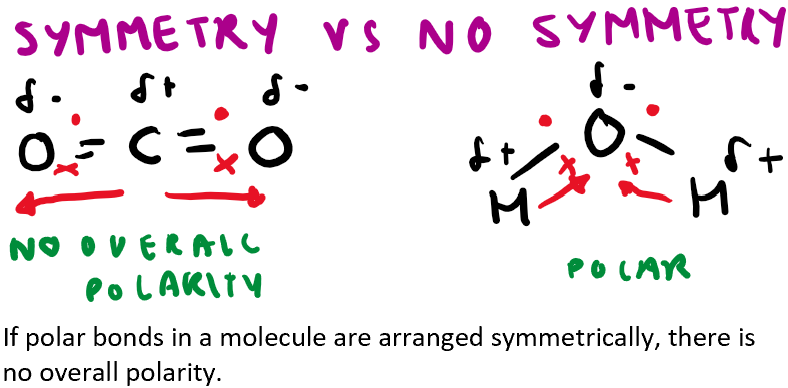
How does symmetry affect the polarity of a molecule?
Symmetry- dipole moments cancel
Assymetry- dipole moments do not cancel