Y11 Chemistry Test 1 - Atomic Structure and Bonding
1/64
Earn XP
Description and Tags
i love you buckminsterfullerene
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
65 Terms
John Dalton’s Theory of the Atom
atoms are indivisible structures
all atoms of a given element have the same properties
compounds are combinations of multiple atoms from different elements
a chemical reaction is a rearrangement of atoms
the arrangement of atoms determine physical properties
based on law of conservation of mass and constant composition
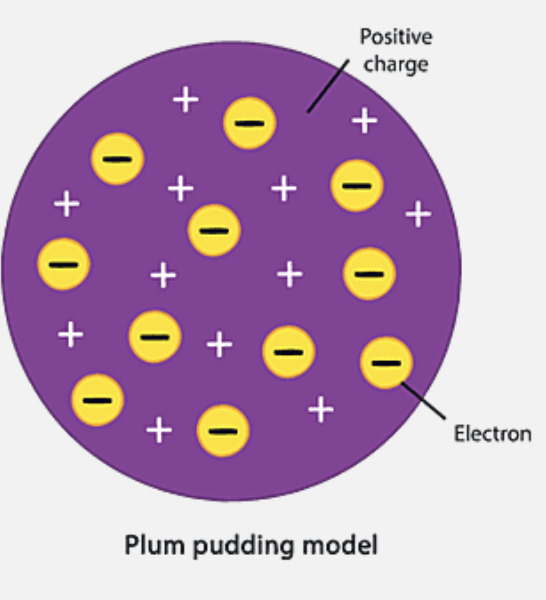
J. J Thomson’s Theory of the Atom
constructed the cathode ray tube
glass tube, emptied of air, which contained two sheets of metal, one positively charged (cathode) and one negatively charged (anode)
found that particles would flow from the negatively charged to the positively charged plate, making them negatively charged particles
also found that they moved very easily from magnetic fields, meaning that they were of very low mass
further discovered this occurred in all elements, meaning electrons were present in all elements
called these particles electrons, theorised they were embedded within a large positive filling/sphere thing
E. Rutherford’s Theory of the Atom
Conducted Gold Foil Experiment
Assumed that due to Plum Pudding Model, atoms were low density high volume structures, and thus alpha particles would just knock away the atoms
Shot alpha particles at a very thin piece of gold foil, discovered that most passed right through, and a small few were completely bounced back
theorised atoms have a dense positive centre surrounded by orbiting electrons
Bohr’s Theory of the Atom
Explained atoms emitting specific wavelengths of light as atoms moving to a higher energy level
Theorised electrons would absorb the required amount of energy to move to that higher level
As electrons always absorbed specific amounts of energy, the paths must be very well defined
Believed that the atom was made up of a positive core surrounded by fixed paths of electrons
Chadwick’s Theory of the Atom
Explained the unaccounted for weight that wasn’t taking up by the protons or electrons
shot alpha particles at a sheet of beryllium, causing an unknown particle to be released, which was unaffected by magnetic fields or charged, making it neutrally charged
When this neutral particle was shot at paraffin wax, it knocked away protons, making it highly penetrating
believed they were basically the same weight as protons, and named them neutrons
Structure of Atom
Made up of:
Nucleus
Central part of atom that has very low volume, but is highly dense
Contains protone and neutrons, similar mass\
Electron Cloud
Makes up the majority of the volume within the atom
Takes up very little of the mass
Contains electrons
Isotopes
Variations of atoms which have the same amount of protons, but differing amounts of neutrons. Share the same chemical properties with other atoms of the same element, but different physical.
Each variation takes up the same proportion of the element throughout all substances
amu
atomic mass unit, equal to 1/12th of a carbon-12 atom, and is the unit for Relative Atomic Mass
Mass Spectrometer
A device used to measure the distribution of isotopes within a sample, in order to find the RAM of an element, and is made up of four parts, the ioniser, accelerator, deflector and detector
Ioniser
First part of the mass spectrometer that a sample goes through. Ionises a vapourised sample, converting them into cations (usually +1 charge)
Accelerator
The second part of the mass spectrometer that a sample goes through. Uses a negative and positively charged plate in order to accelerate and attract the cations toward the negative plates. Ions move through a small slit in the cathode, forming a single stream of positively charged particles
Deflector
Third part of MS that particles move through. Uses magnetic fields of varying intensities to move the streams toward the detector. As lighter isotopes are affected more by the magnetic field, thereby splitting the positive stream into multiple
Detector
Final part of the MS that a sample goes through. Measures the amount of collisions that occur on it for each stream of a certain weight of isotopes.
RAM Calculation Formula
(each isotope mass times their abundance) ÷ (the sum of all of the abundances)
Order of Electron Config
use data sheet
s (1 per energy level), p (3), d (5), f (7)
1s2, 2s2 2p6, 3s2, 3p6, 4s2, 3d10 so on so on
Ground State
The state of electrons that uses as little energy as possible by going to the closest possible position to the nucleus as possible
Excited State
The state of electrons where they absorb energy (heat, light, or electrical), causing them to move to a higher energy level (this is unstable). Upon return to ground state, a specific wavelength of light is released corresponding to the energy absorbed
Flame Tests
Tests in which metals are put into a flame in order to identify the metal based on the colour of the flame. When the metals is put in the fire, the electrons of the metal absorb the heat energy, causing them to move to a higher energy level. Once the electrons return to ground state, they will release a specific wavelength of light corresponding to the energy absorbed into the fire, causing the flame to change colour
Atomic Emission Spectroscopy
Process used to create the Atomic Emission Spectrum of an element. Excites the electrons of a substance by giving them sufficient energy to increase in energy level. Once they return to ground state, that element will release specific wavelengths corresponding to the energy absorbed, which are unique to that element. A prism can be used to separate all of the wavelengths, and then be recorded. This can be used to identify an element as each element has a unique AES
Atomic Absorption Spectroscopy
A device used in order to identify the concentration of a substance. Shoots the specific wavelength of light that excites a certain element, allowing them to identify if the element is present, and how much is present based on how much is absorbed. When an element absorbs light, it releases it in all directions, causing it to be weaker in one direction. Is made up of several parts, including a hollow cathode lamp, nebuliser/vapouriser, monochromator, and detector.
Hollow Cathode Lamp
Lamp that shoots the specific wavelength of light required to excite the element being identified. Is often coated with that element.
Nebuliser/Vapouriser
Part of AAS which converts the sample into a gas, allowing the wavelengths to pass through the vapour and be absorbed by electrons if the element is present
Monochromator
Part of AAS which is used to filter light. Removes all light going towards detector of a different wavelength than the specific one being studied
Detector
Final parts of the AAS. Measures the amount of the specific wavelength received compared to the amount emitted by the hollow cathode lamp. If:
all of the light passes through, the substance is not present
If only some of the light passes through, the substance is present (because the substance would emit it in all directions, causing less to go in one specific direction)
Absorbance
The measure of how much light is absorbed, and is used in AAS. The higher this is, the less light gets through
Calibration Curve
The line used to find the concentration from the absorbance given in the AAS. Is made by putting 5 or more known samples into the AAS, and using the absorbance found to plot them on a graph, and then create the line of best fit. From there, you can use the other absorbance levels to find where concentration they correspond to on the line of best fit
Core Charge
The net positive charge experienced by the valence electrons. Is found by subtracting the total number of protons from the number of non-valence electrons. Increases as you go right on the periodic table
Number of Energy Levels
The number of energy levels within an atom, creating distance between the valence electrons and the nucleus. Increases as you go down the periodic table
Electronegativity
The tendency of an atom to attract the electrons from other atoms. Increases with core charge as higher positive attraction means a stronger pull on the negatively charged electrons. Decreases with energy levels as distance from the nucleus weakens the pull from the protons
Atomic radius
The radius of an atom, or the distance between the centre and the outermost electrons. Decreases with core charge as higher attraction means greater pull on electrons, attracting them closer. Increases with energy levels as distance from nucleus means a weaker pull, meaning electrons are not pulled as closely to the nucleus
First Ionisation Energy
The amount of energy required to remove the first electron from an atom. Increases with core charge due to higher positive attraction meaning a greater pull on electrons and thus require more energy to remove. Decreases with energy levels as more distance from the nucleus causes a weaker pull on electrons, requiring less energy to remove
Metallic Properties
How metallic a substance is. Decreases with core charge due to metals generally having a low amount of valence electrons. Increases with energy levels
Successive Ionisation Energy
The amount of energy required to remove each successive electron from an element. Proved the existence of energy levels as the amount of energy required did not follow a linear fashion. Instead, would have sections of moving linearly broken up by large jumps. These jumps indicate a sudden increase in proximity to the nucleus, proving that electrons are found in groups, where groups are in varying distances from the nucleus
How Atoms Become Most Stable
When they achieve a full p orbital in their valence shell, causing them to be unreactive. Atomic properties will affect how this is achieved
The 4 Properties that indicate atomic arrangement
Malleability
Conductivity in Solid Form
Conductivity in Molten/Aqueous Form
Melting/Boiling point
Malleability
The ability of a substance to change shape, permanently, without breaking the bonds between particles. Relies on particles having non-directional bonding between them, meaning bonds can exist in all directions
Electrical Conductivity when Solid and Molten/Aqueous
The ability of a substance to carry and transport charges throughout it, which relies on the presence of mobile charged particles. This property can sometimes only be present in certain states, though this is not always the case
Melting/Boiling Point
The amount of thermal energy required for a substance to change states, which depends on the strength of the bonds between particles. The stronger the bonds are between particles, the more energy is required to overcome them
Metallic Bonding
The bonding that occurs between metals. Occurs when many atoms of a metal are together, and release their electrons past their p orbital, allowing them to move freely between the metallic ions. Forms a lattice structure of metallic ions all attracted by a sea of delocalised valence electrons
Properties of Metallic Bonds
Are electrically conductive when both solid and molten/aqueous due to the sea of delocalised electrons (and metal ions when liquid) being able to act as mobile charged carriers
Are malleable due to the electrons that attract metal ions being freely moving, meaning that when metal ions move, the electrons can still attract them
Have high melting/boiling points due to the high electrostatic attraction between the sea of delocalised valence electrons and positive metal ions which requires a large amount of energy to overcome
Ionic Bonding
Bonding between (generally) a metal and non metal, where highly electronegative non-metals attract the low ionisation energy metals, allowing each element to achieve a full p orbital in their outermost energy level. Creates a lattice of alternating positive and negatively charged ions, with a strong electrostatic attraction between particles
Ionic Bonding properties
Brittle, due to force applied causing like charged particles to align, and repel each other, breaking apart the substance
Not electrically conductive when solid, as despite all particles being charged, there are no mobile particles as they are all kept in place by strong electrostatic bonds
Electrically conductive when molten/aqueous as both the positive and negative ions become mobile, allowing them to transport charged
Very high melting and boiling point due to an incredibly strong electrostatic attraction between the anions and cations, requiring much energy to overcome
Ion Formation
The process in which atoms give and take electrons so that each can achieve full p orbitals in their outermost energy level. The amount given and taken must be equal, allowing you to find the ratio of atoms
Ionic Formula
The formula used to work out the ratio of cations to anions.
Steps include
Find the magnitude of the charges of each atom when ionised
Find the lowest common multiple
LCM/charge = number of atom
process is the same with polyatomic ions, just treating a polyatomic as one body
Electron Dot Diagrams for Ionic Substances
Used to show the amount of electrons in the highest energy level. You create by:
Writing out each ion symbol
Drawing the amount of electrons in the outermost energy level around it, differentiating between the regular electrons and those taken from the metal
surround in square brackets, and place charge in top right corner
Give a coefficient if more than 1 of that ion within the bond
Ionic naming scheme
Use the ion naming sheet, put cation first, then anion second
Covalent Molecular Bonds
Bonds where two or more non metals share electrons in order to achieve full p orbitals. Each group of atoms connected by bonds is known as a molecule. Each molecule has very strong intramolecular forces between covalently bonded atoms, but very weak Van der Waals charge [non-directional] between each molecule (intermolecular forces). As well as this, each molecule is neutrally charged
Covalent Molecular Properties
Malleable due to the weak Van der Waals forces being non-directional, allowing molecules to move around each other
An insulator when solid and liquid due to the molecules, despite being mobiles, being neutrally charged
Low melting and boiling points due to the weak Van der Waals charges between molecules requiring little energy to overcome
Covalent Molecular Naming Scheme
Elements toward the top and left of the periodic table are generally put first in the name. The first element in the name does not include a number prefix unless there is more than one of it. The second element ends with ide usually, and includes a prefix indicating amount, even if there is just one
e.g., PCl3 → Phosphorous Trichloride
Covalent Electrons Dot Diagrams
Are made with the following steps:
Find the total number of electrons an element has in it’s outermost energy level
Find the total number of electrons needed to achieve a full p orbital
Draw out atoms with dots that correspond for each electron needed, and connect each dot to each other to form one structure
Retract lines between dots so that each atom is next to each other
Convert each line into a pair of electrons
Draw all of the non-bonding valence electrons in as well
Coordinate Covalent Bonds
Bonds between atoms where the amount of electrons shared by each atom is not equal, commonly with one atom sharing none.
Non-Octet Rule Elements
Elements which in certain circumstances do not require 8 electrons to fill their outermost energy level.
e.g.,
B = 6
N = 7
P = 10
S = 12
Polyatomic Ions
Ions that contain multiple atoms. Occur in acids when they lose hydrogen atoms
Steps into finding electron dot diagram to polyatomic ions from acids
Add a hydrogen atom for each negative charge
Identify the central atom (not a H or O)
Connect a HxOOo (o and x representing electrons, not number) to the central atom for every hydrogen
Check for the central bond’s config
if stable, add any extra oxygens as coordinate covalent
if not, add extra oxygens and introduce double bonds
Remove the hydrogens added at beginning to return to original form
Add square brackets and charge
Carbon monoxide Structure
CO
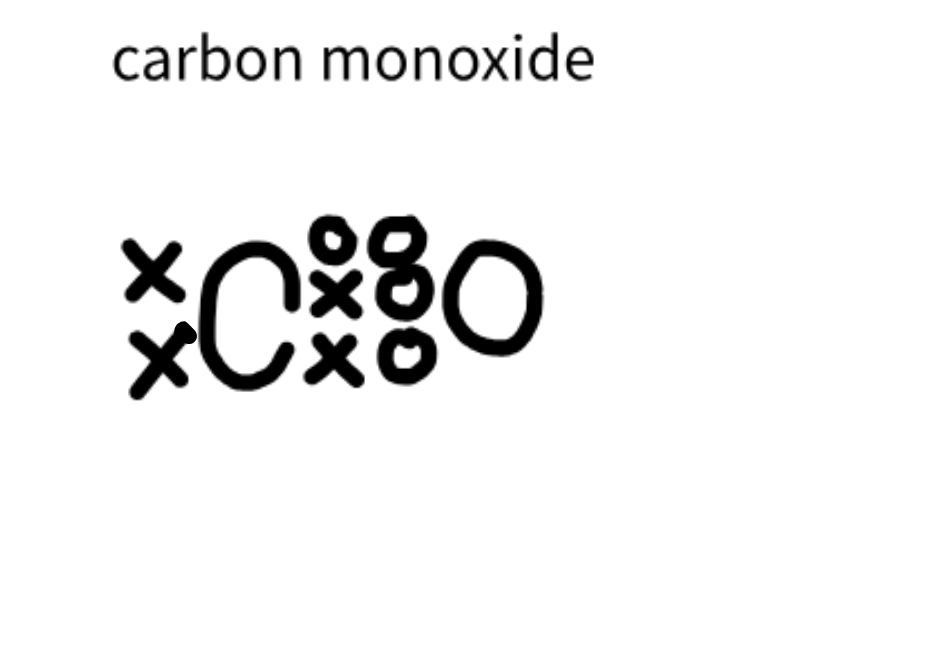
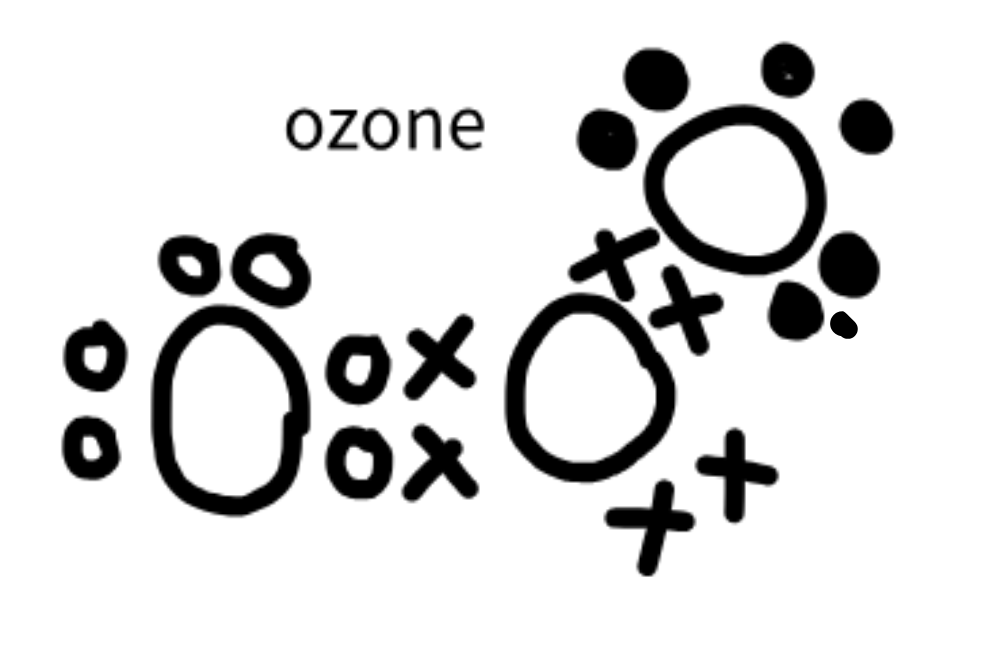
Ozone Structure
O3
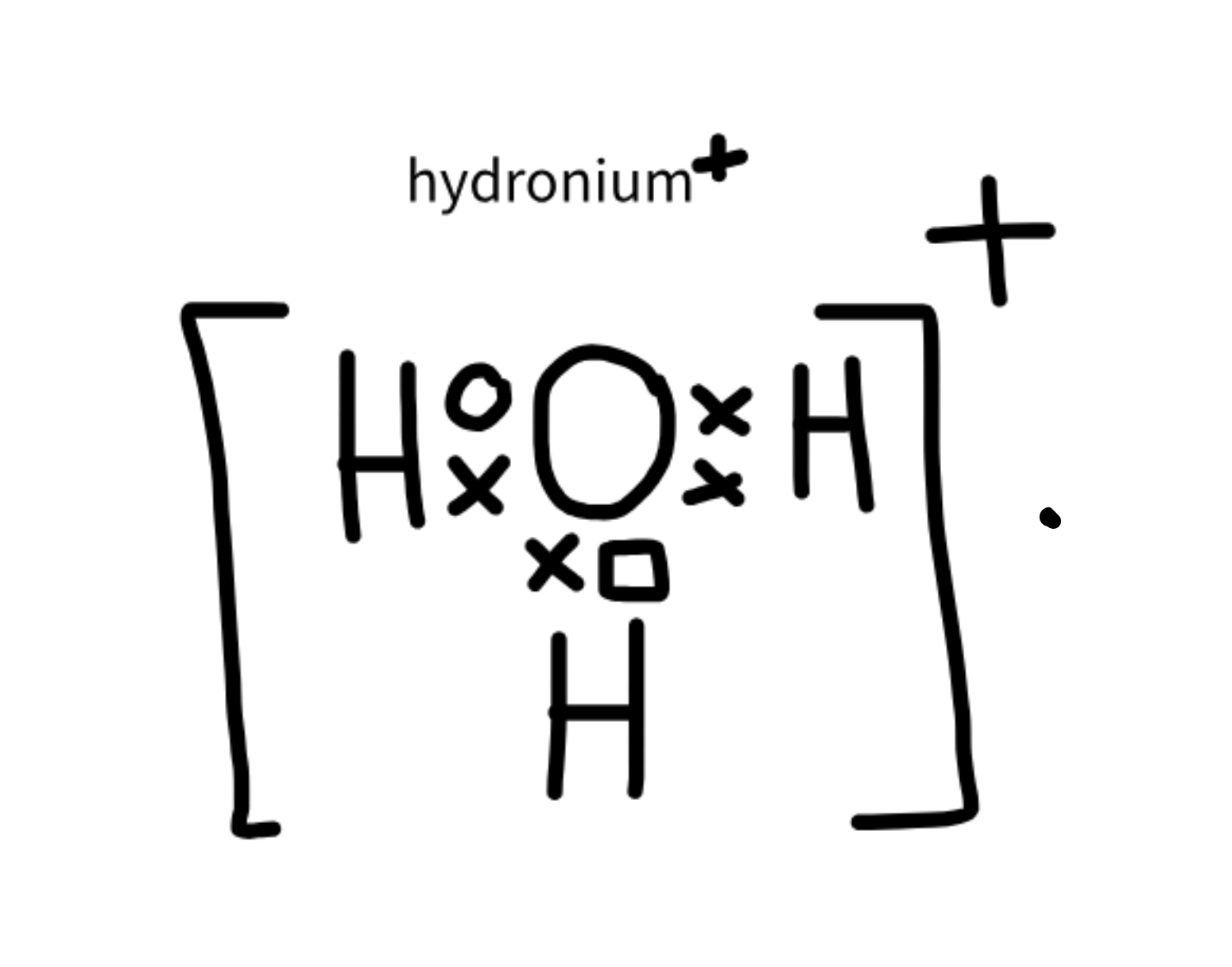
Hydronium
H3O+
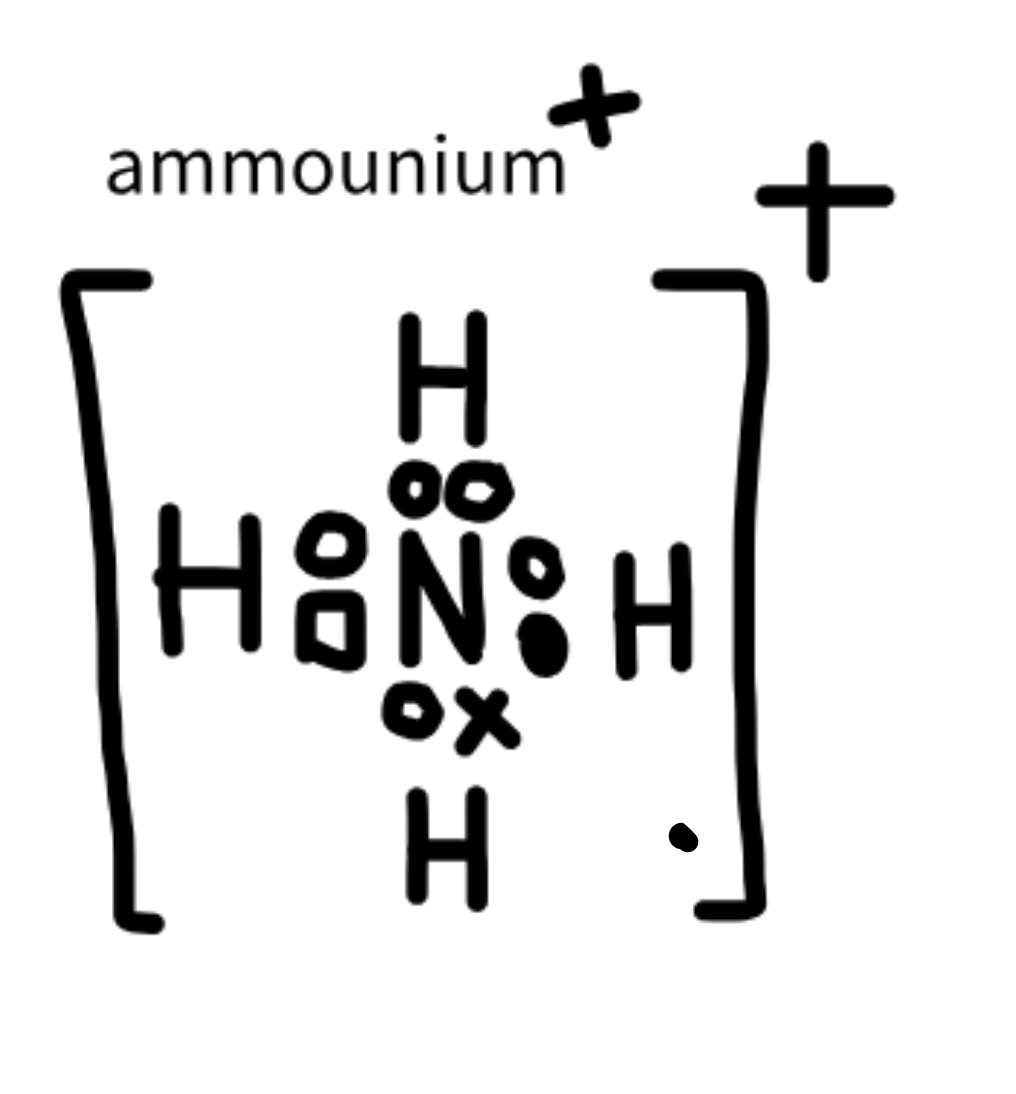
Ammonium
NH4+
Covalent Network Substances
Substances made up of atoms that are covalently bonded together, not in groups, but in one large network of atoms intramolecularly bonded. Are generally made up of Group 14 Elements as they can make 4 covalent bonds
Covalent Network Properties
Generally not malleable due to the intramolecular forces and network structure not allowing atoms to move very far before overcoming the covalent bond
Generally high melting/boiling point due to the strong covalent bonds between atoms requiring a lot of energy to overcome
Generally not conductive as each atom would be immobile and thus unable to transport charges
Carbon Allotropes
The several different covalent network substances carbon can form, depending on the conditions carbon experiences. There are 4 of these:
Diamond
Graphiye
Buckminsterfullerene
Amorphous Carbons
Diamond
An allotrope of carbons, where each carbon is bonded to another carbon in a tetrahedral arrangement (like the vertexes of a pyramid), which is highly stable and makes diamonds very hard. Are brittle, non-conductive, and have high melting and boiling points
Graphite
An allotrope of carbon, where each carbon atom is bonded to 3 others in a hexagon shape, making up individual sheets (known as graphene). There are weak Van der Waals charged between each sheet, making this allotrope slippery as the sheets can easily move around independent of one another. Intramolecular forces within sheets are very strong, requiring a lot of energy to break the bonds between carbon atoms.
conductive when solid due to 4th electron being freely moving between sheets of graphene
high melting and boiling point due to strong bonds
Brittle because of non-directional bonding
Buckminsterfullerenes ❤
An allotrope of carbon, made up of carbon atoms bonded to three other carbons, creating hollow spheres of varying sizes. Is very slippy due to weak Van der Waals charges between balls, allowing them to roll over one another, making this a dry lubricant.
High melting/boiling point due to strong bond between each carbon requiring much energy to overcome
Not malleable due to covalent bonds not being able to move too far without breaking
Not conductive, despite the 4th electron being delocalised, as the electrons are localised within the structure, and thus are unable transfer charges between fullerenes
Amorphous carbon
An allotrope of carbon where the carbon atoms are randomly bonded to one another without a consistent structure (e.g., coal, soot). Lack of uniform structure results in being quite weak.
Not conductive due to a lack a consistent delocalised valence elctrons
Brittle due to the non-uniform structure and lack of non-directional bonding due to covalent bonds
High melting and boiling point due to very strong intramolecular forces requiring much energy to overcome