Chapter 24, Lesson 2: Electrolyte Balance
1/20
Earn XP
Description and Tags
Flashcards from Chapter 24, Lesson 2 of McGraw Hill Anatomy and Physiology, Tenth Edition, by Kenneth S. Saladin.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms
Electrolyte balance
The amount of electrolytes absorbed by the small intestine balances the amount lost in urine
Electrolyte functions
Metabolic reactions
Membrane electrical potential regulation
Osmolarity adjustment
Water content regulation
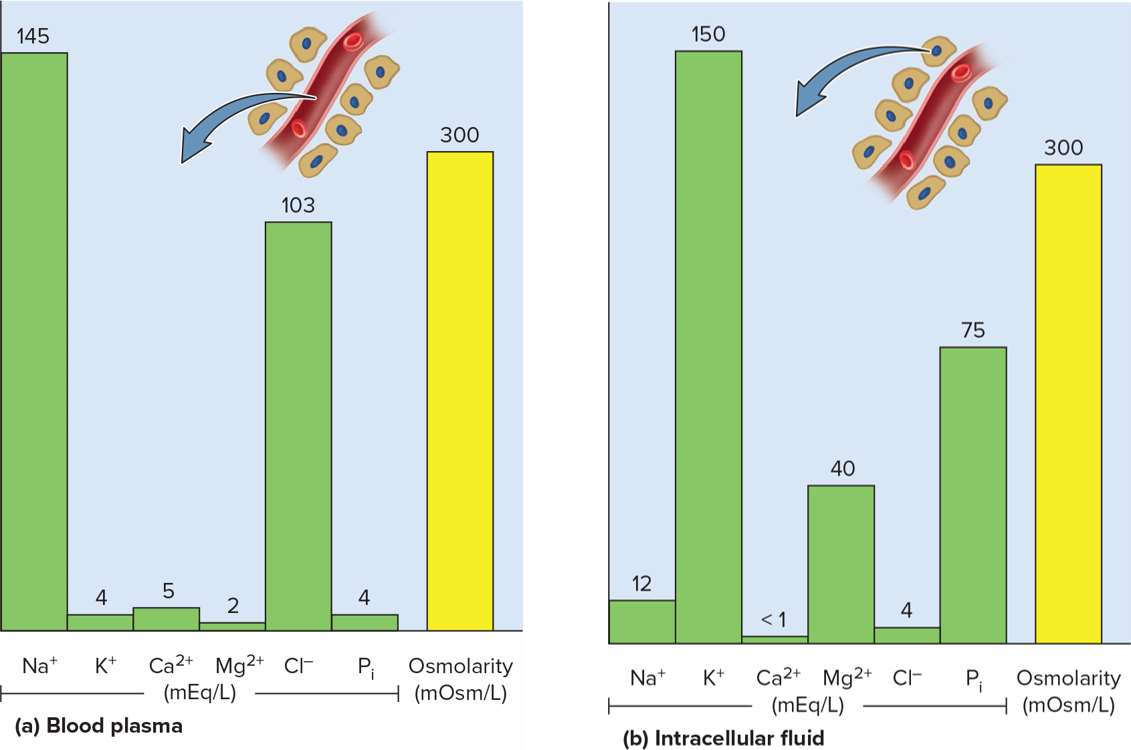
Sodium
The principal cation in the ECF that signals nerve and muscle cells while regulating hydration
0.5 grams
The amount of sodium an adult needs per day
3 to 7 grams
The typical sodium intake of an American adult; caused by excessive preservatives
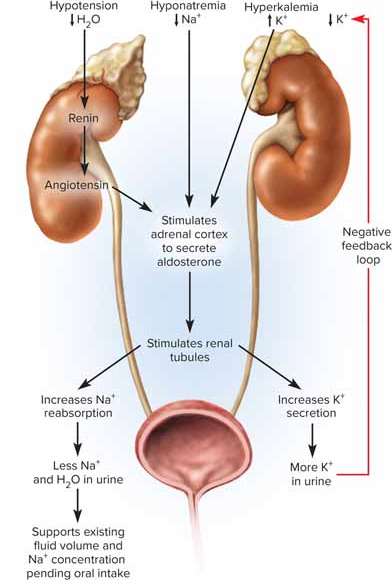
Aldosterone
Hormone that plays the primary role in adjusting sodium excretion
Antidiuretic hormone (ADH)
Hormone that modifies water excretion independently of sodium excretion; higher sodium levels lead to more retention
Hypernatremia
High sodium levels greater than 145 mEq/L; can result in water retention, hypertension, and edema
Hyponatremia
Low sodium levels lower than 130 mEq/L; can be caused by excess body water and is followed by rapid excretion through sweat or urine
Potassium
The principal cation in the ICF that signals nerve and muscle cells; linked to sodium homeostasis but with dangerous imbalance effects
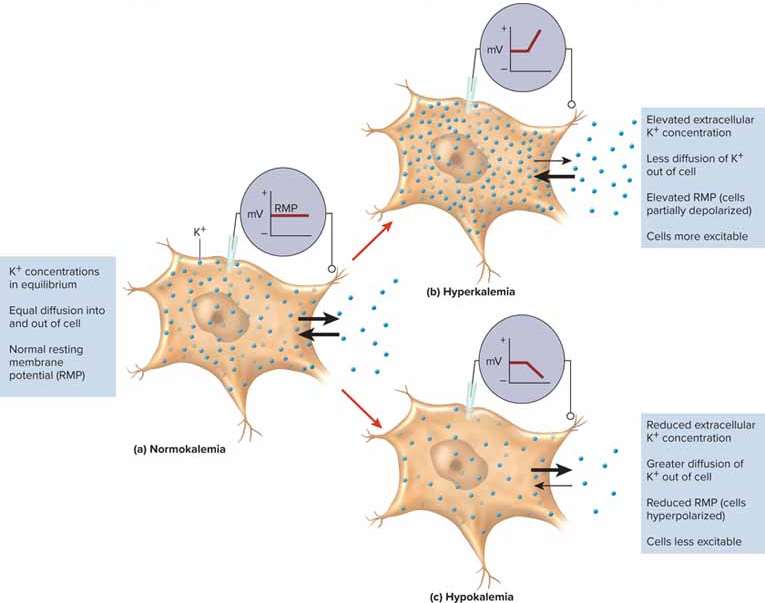
Hyperkalemia
High potassium levels above 5.5 mEq/L; can lead to overexcitation and cardiac arrest
Hypokalemia
Low potassium levels under 3.5 mEq/L; can be caused by excessive fluid loss and leads to underexcitation and weakened muscles (including the heart)
Calcium
Electrolyte that functions in skeleton strength, muscle contraction, neurotransmission, and blood clotting
Hypercalcemia
High calcium levels over 5.8 mEq/L that inhibits depolarization of nerve and muscle cells; can cause weakness, depressed reflexes, cardiac arrythmias
Hypocalcemia
Low calcium levels under 4.5 mEq/L that increases excitability; can lead to tetany, laryngospasm, death
Magnesium
Electrolyte that aids in ATP and intestinal absorption
Hypermagnesemia
High magnesium levels
Hypomagnesemia
Low magnesium levels
Chloride
The most abundant anion in the ECF required for stomach acid formation and body pH regulation
Hypochloremia
Low chloride levels that can result from hyponatremia, hyperkalemia, or acidosis
Hyperchloremia
High chloride levels that can result from dietary excess