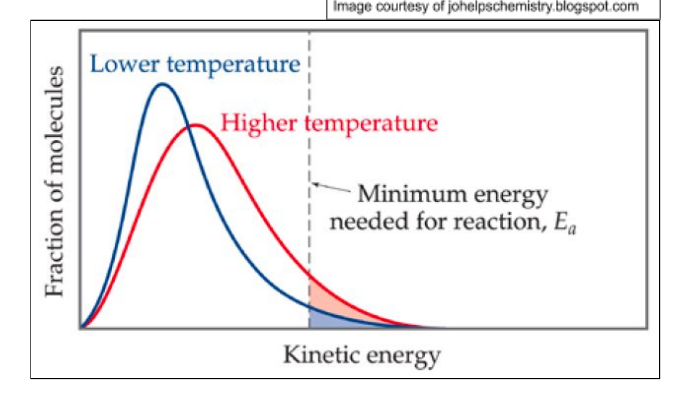
Effect of Temperature
0.0(0)
0.0(0)
Card Sorting
1/4
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
5 Terms
1
New cards
substance heated =
thermal energy transferred to is
-converted to kinetic
-molecules of substance move faster and further
2
New cards
increased movement of molecules =
collisions occur more often with greater energy = reaction occurs
3
New cards
increasing temperature = increasing rate of reaction as
more collisions of greater energy occur in a given time
4
New cards
increase in temperature = maxwell boltzmann distribution shifts:
right
-greater proportion of molecules have energy greater than or equal to activation energy
5
New cards
Maxwell Boltzmann - increase in temperature