IR Spectroscopy
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
26 Terms
Alcohol O-H
3200-3600 (Broad)
Carboxylic acid
2200-3600 (Very broad)
N-H bond
3350-3500
Hydrogen/triple-bonded carbon
~3300
Aldehyde C-H bond
2750 and 2850 (Two weak signals)
Aldehyde C=O bond
~1730
IR values will be _____ if the C=O bond is conjugated
LOWER
What does “conjugated” mean?
Resonance is possible
C=O double bond (general)
~1650-1820 (strong)
C=C bonds
1600-1700
Benzene rings
1450-1600 / 1650-2000
Carbon-carbon triple bond
2100-2200
CN triple bond
2200-2300
|
— C — H
|
2850-3000
= C — H (note trigonal planar shape)
|
3000-3100
O = C — O — (note trigonal planar shape)
|
1250-1350
|
— C — O —
|
1000-1100
C-N bond
1000-1200
C-Cl
600-800
C-Br
500-600
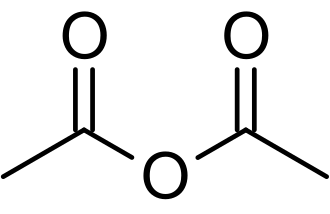
Anhydride, between ~1820 and ~1760
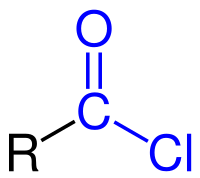
Acid chloride, ~1790
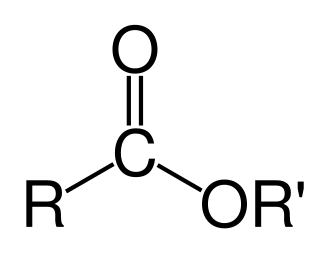
Ester, ~1735
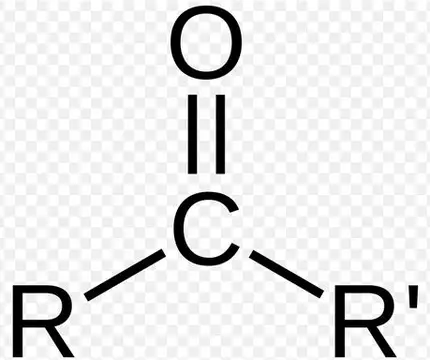
Ketone, ~1720
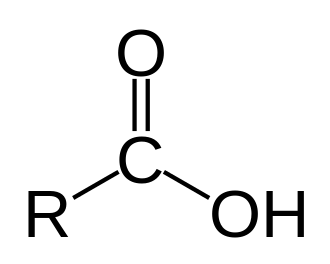
Carboxylic acid, ~1715
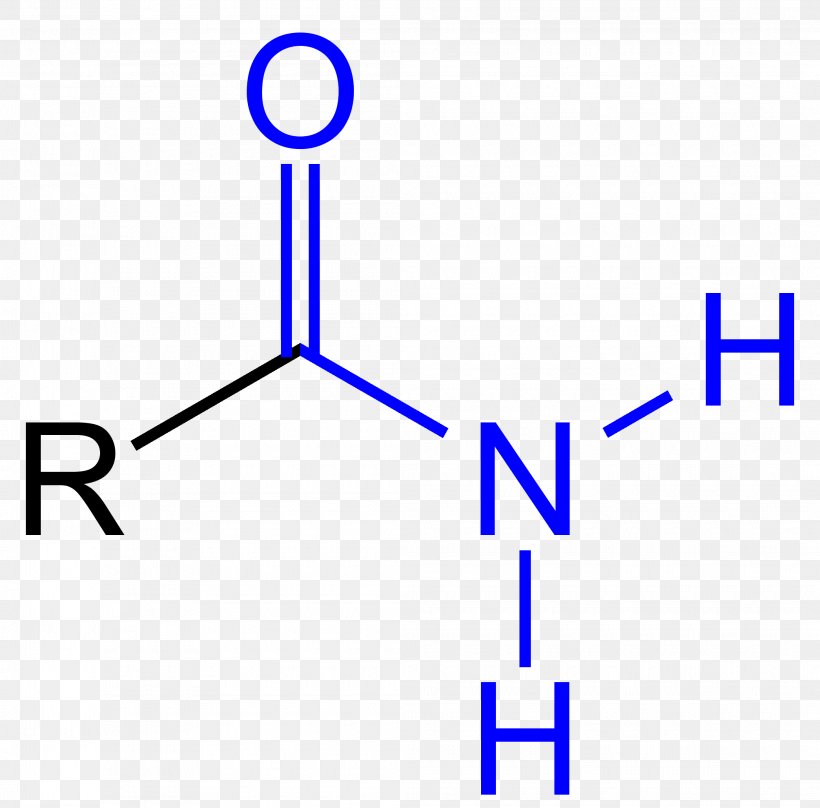
Amide, ~1650