Cell Immortalisation, Telomeres and Cancer Stem Cells
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
29 Terms
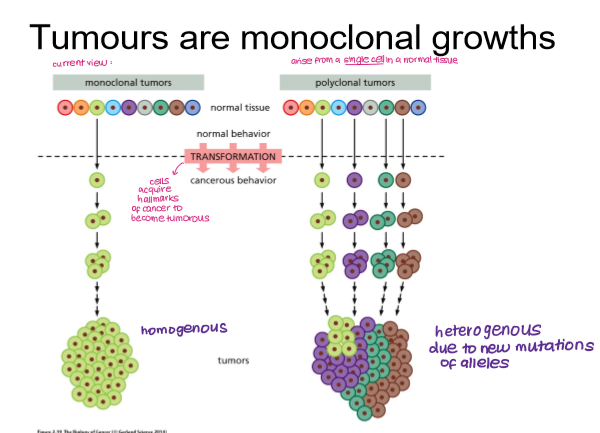
Tumours are ____________ growths. What does this mean?
monoclonal
arise from single cell in a normal tissue, forming a homogeneous tumour that can acquire mutations and become heterogeneous
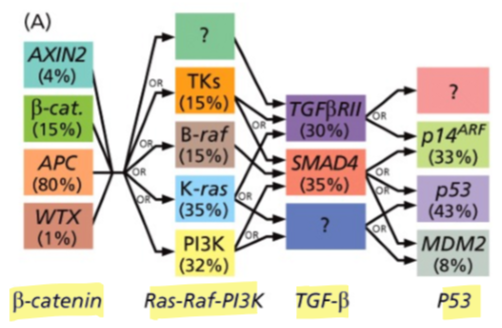
Do cancers usually require one or multiple mutations to drive cancer development?
many mutations affecting various genes
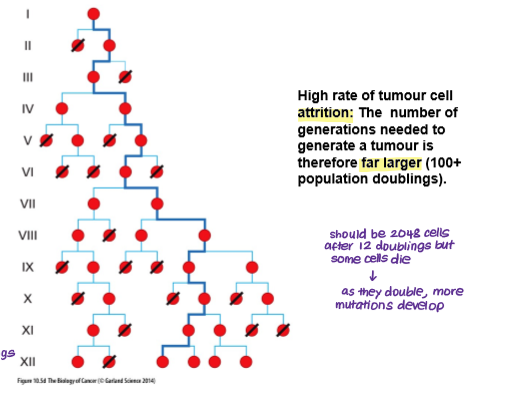
How many cell successive cell generations are required to make a clinically detectable human tumour and how many cells would be found in that tumour?
10^9 → clinically detectable tumour
10^12 → life threatening tumour (40 cycles)
BUT there is a high rate of tumour cell attrition so the number of generations needed to form a tumour is much higher (>100)
What are the two barriers that cancer cells must overcome in order to replicate limitlessly?
Senescence
Crisis
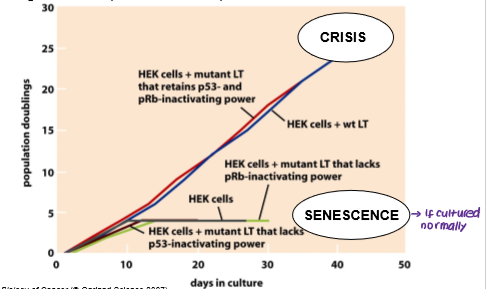
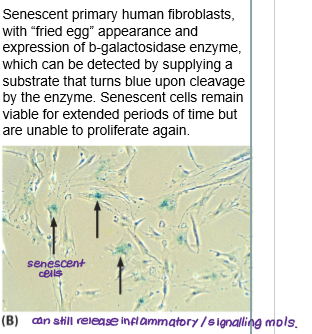
What is senescence?
Cells permanently stop proliferating (cell cycle arrest) due to stress but do not die.
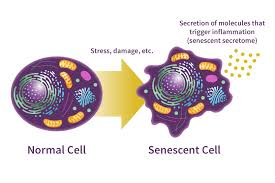
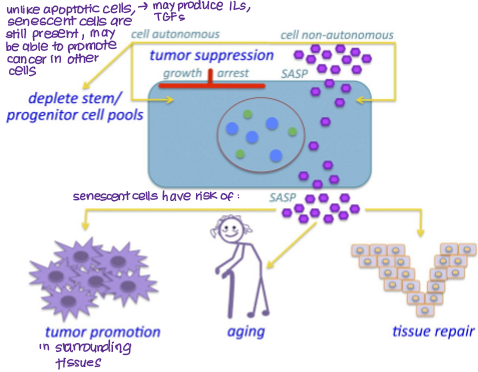
Can secrete inflammatory/signalling molecules (SASP- senescence associated secretory phenotype) that can promote tumour growth, aging and tissue repair.
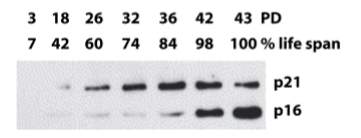
Senescence is typically induced by increased expression of which tumour suppressor genes?
p16 (block phosphorylation of Rb)
p21 (induced by p53 and inhibits all CDKs)
Senescent cells are not just found in culture but have also been observed in living tissues. For example:
Adenoma in colon
Induced by chemotherapy
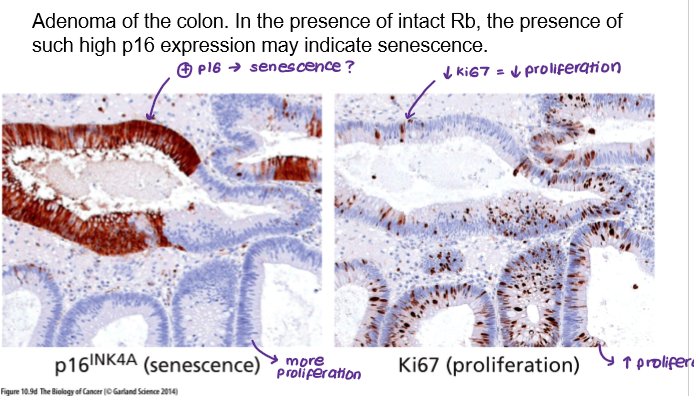
How can senescent cells promote cancer?
SASP (senescence-associated secretory phenotype)
releases signalling and pro-inflammatory molecules (e.g. TGF-b, ILs)
can be autocrine (promote senescence)
can be paracrine (promote tumour development)
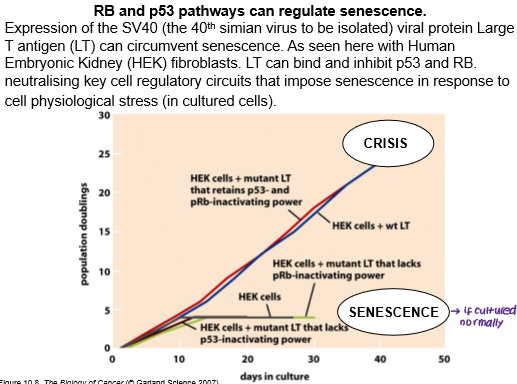
Even if p53 and Rb are inhibited, allowing cells to escape senescence, cancer cells must also overcome crisis in order to replicate limitlessly. What is meant by crisis?
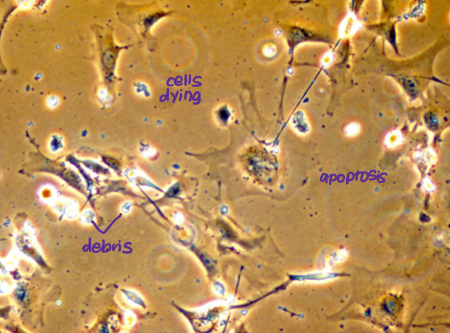
State when cells lose telomeres of adequate length, resulting in end-to-end fusion of chromosomes, genetic chaos and widespread apoptosis.
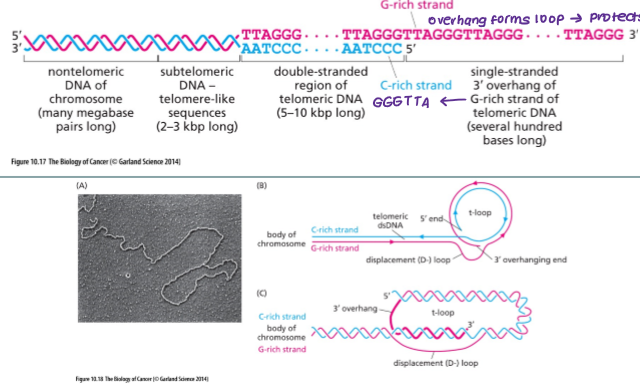
What are telomeres?
A telomere is a region of repetitive DNA sequences at the end of a chromosome, forming a capped end structure that is associated with a T-loop. Telomeres protect the ends of chromosomes from becoming frayed or tangled and shorten with each division.
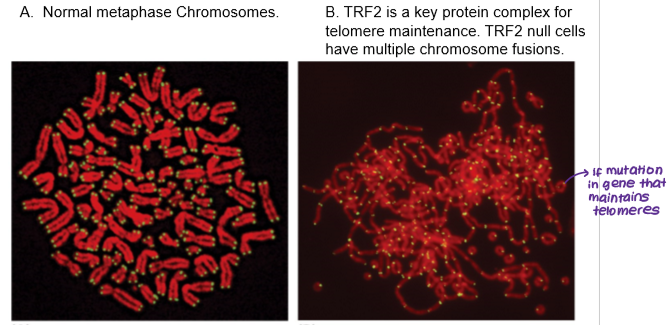
Telomeres are associated with which sequence on the 3’ DNA strand?
GGGTTA
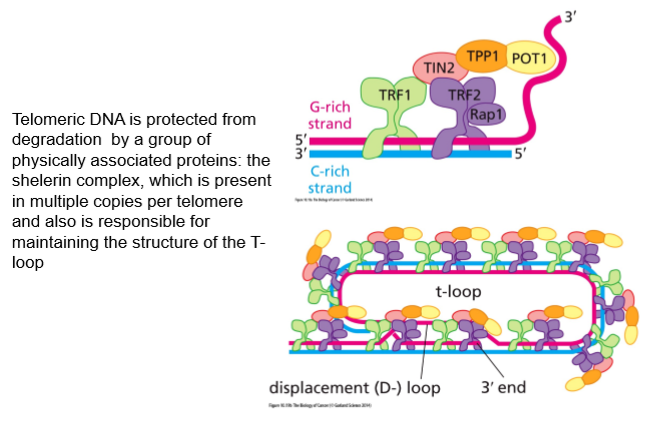
Telomere maintenance is regulated by which genes?
Multiple telomere specific proteins bind to telomeric DNA:
TRF1
TRF2
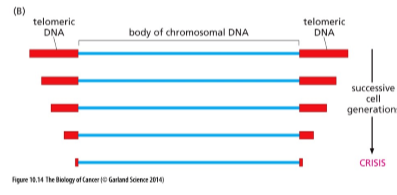
What happens to normal cell telomeres during cell proliferation?
In normal cells:
telomeres shorten with each division because the replication machinery is ineffective at copying telomeric sequences
eventually, telomeres are too short to sustain proliferation
cells enter crisis → apoptosis
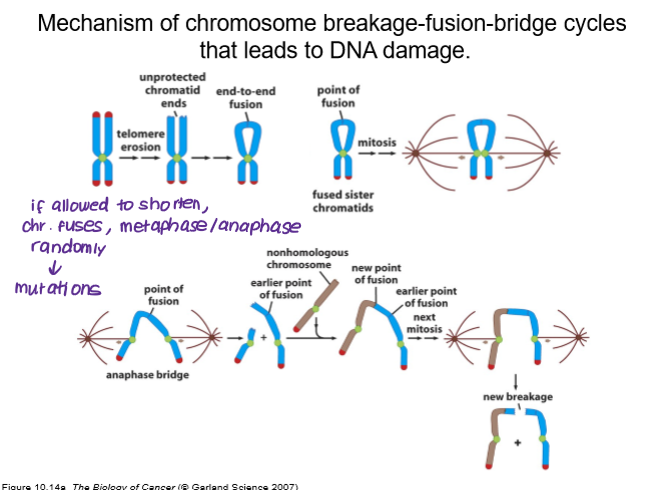
How do shortened telomeres lead to DNA damage?
breakage-fusion-bridge (BFB)
telomere erosion leads to unprotected chromatid ends
end-to-end fusion of chromosomes
random anaphase created new points of fusion
Breakage: A telomere is lost or broken, often due to critically short telomeres, leading to unprotected chromosome ends.
End-to-end Fusion: During replication, the unprotected, broken ends of sister chromatids fuse together, creating a dicentric chromosome (a chromosome with two centromeres).
Bridge: In anaphase, the two centromeres are pulled to opposite poles, forming a bridge of DNA that stretches across the dividing cell.
Breakage (Cycle Repeats): The bridge breaks under tension, often unevenly, resulting in daughter cells with missing or extra chromosomal segments.
How do cancer cells escape crisis?
express telomerase- maintains length of telomeres
human telomerase reverse transcriptase (hTERT) in humans
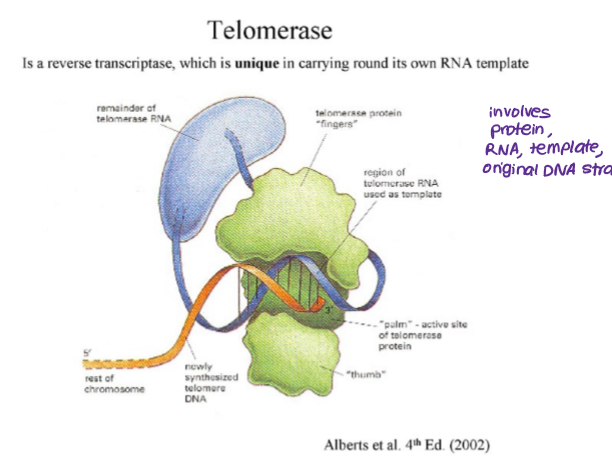
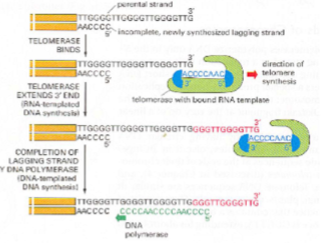
How do telomerases work?
reverse transcriptase- carries own RNA template
uses RNA template to add complementary DNA bases to the end of the chromosome
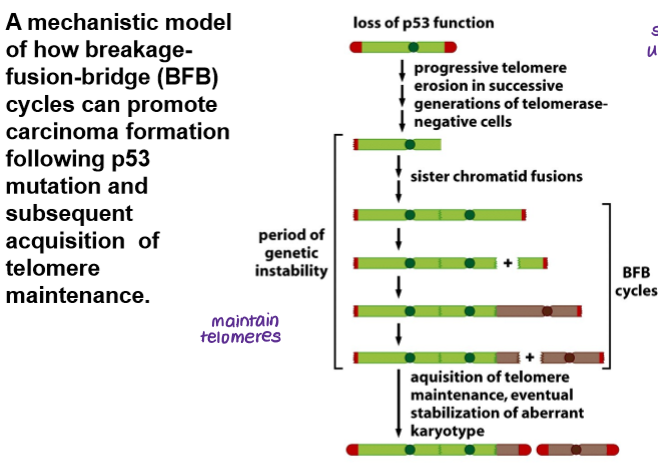
Loss of which gene can increase telomere erosion and thus the BFB cycle?
p53
Which proteins have been linked to increased telomerase (hTERT) expression?
Myc
E6
promote cancer
Which proteins have been linked to repressed telomerase (hTERT) expression?
Menin (from MEN1 tumour suppressor gene)
p53
Mxd
What are the key characteristics of normal stem cells?
rare cells found in an niche
not terminally differentiated
capacity for self-renewal
daughter cells may become a stem cell or a terminally differentiated cell
can divided limitlessly (infrequently)- it is usually the daughter cells that divide limitlessly
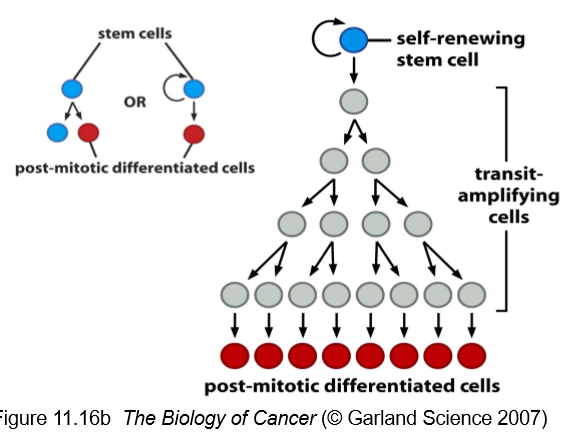
What are the different classes of stem cells?
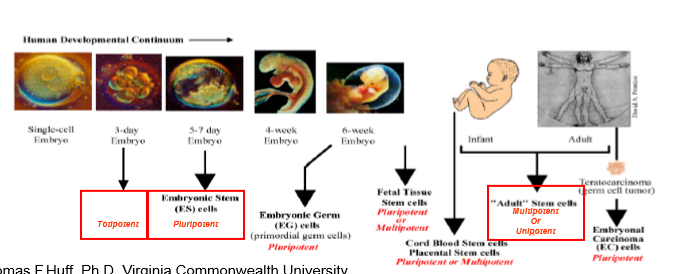
Embryonic (totipotent → pluripotent)
Adult (multipotent)
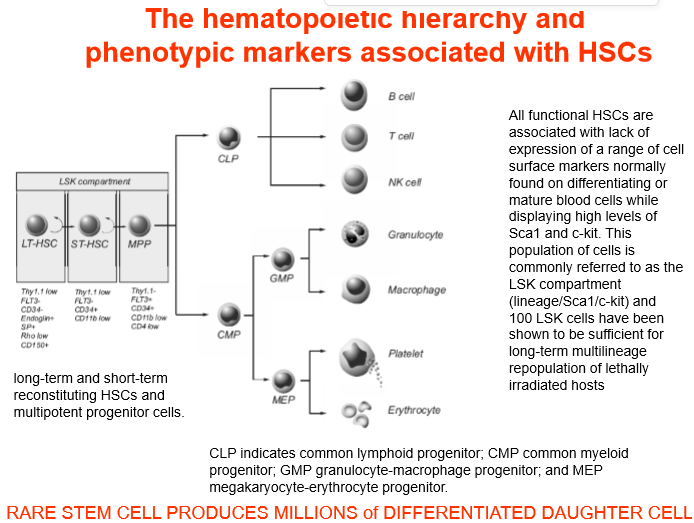
A common example of stem cells in the body are
HSCs
long term and short term HSCs can repopulate entire cell population
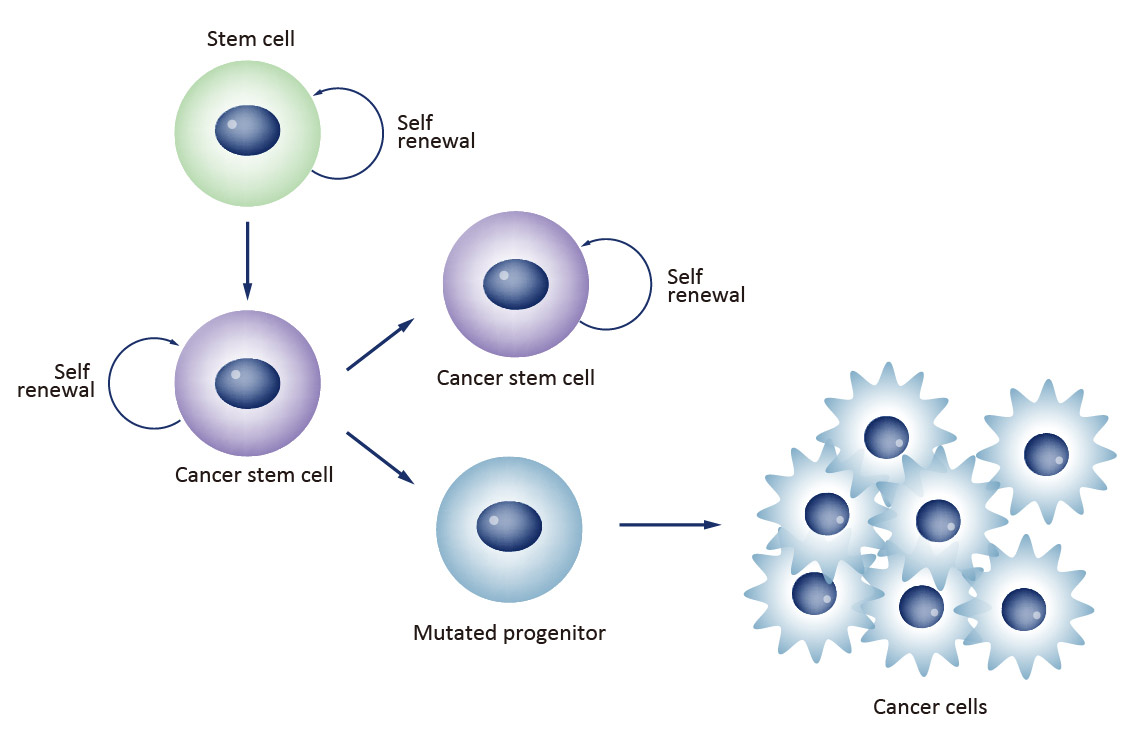
What is the cancer stem cell hypothesis?
Rare cells within tumours have the ability to self-renew and give rise to phenotypically diverse tumour cell population to drive tumorigenesis.
CSCs are malignant cells that display “stem like” properties of self-renewal and ability to produce phenotypically diverse daughter cells.
Cancer stem cells have been shown to be a fundamental cause of which cancers?
acute myeloid and lymphoid leukaemia
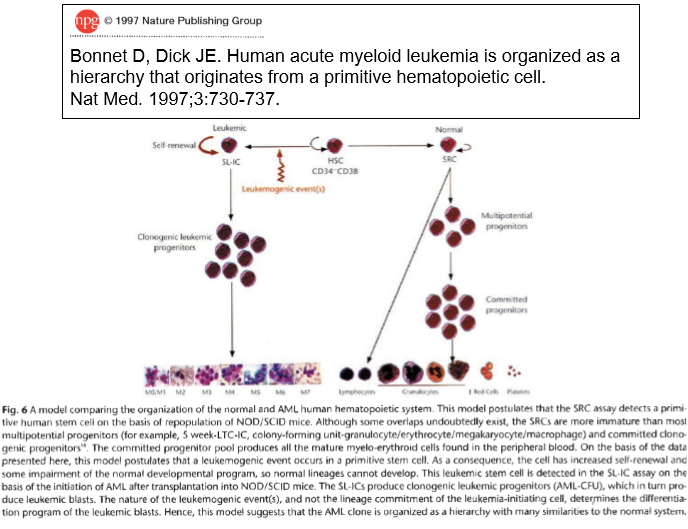
Cancer stem cells may also be the driving force behind tumour formation in solid tissue cancers (e.g. prostate, colon, breast). However, evidence for this is not as clear-cut as it is for haematopoietic cancers because?
no specific stem cell markers for solid tumours (e.g. CD44 and breast cancer)
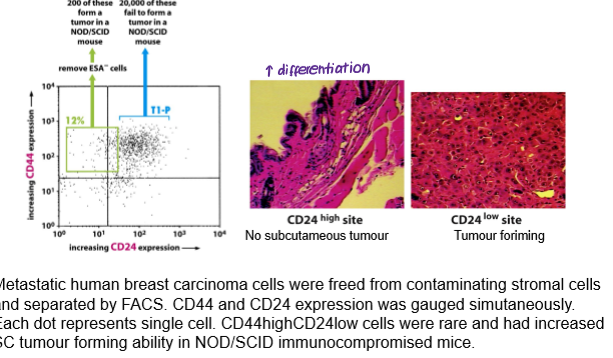
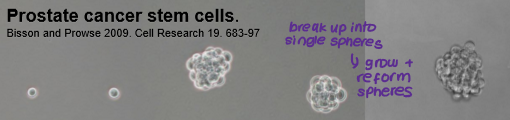
Cancer stem cells may also be the driving force behind tumour formation in solid tissue cancers (e.g. prostate, colon, breast). However, evidence for this is not as clear-cut as it is for haematopoietic cancers. What can be used instead to study cancer stem cells in solid tumours?
clonogenicity assays: ability of cells to form self-renewing colonies in adherent culture conditions.
sphere assays: ability of cells to form self-renewing colonies in non-adherent culture conditions.
tumorigenicity assays: ability of cells to form tumours in immunocompromised individuals
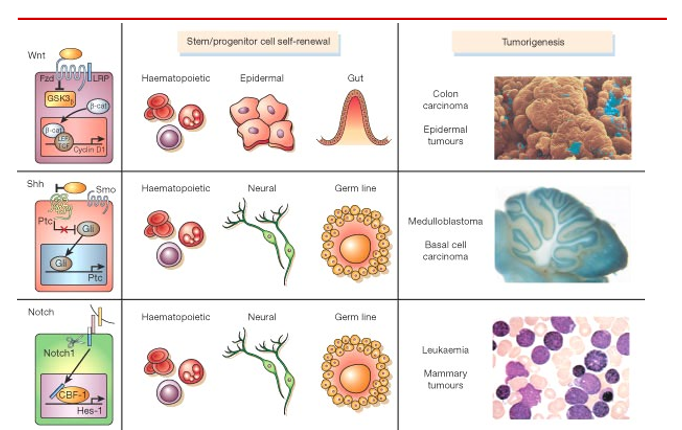
Signalling pathways involved in normal stem cell self-renewal can regulate and self-renewal and proliferation of cancer cells with stem cell characteristics. For example:
WNT
HH (hedgehog)
Notch
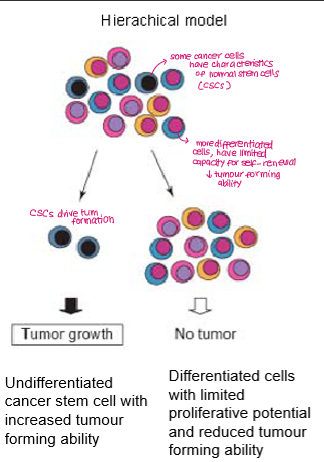
Summarise the cancer stem cell model
Tumours are hierarchically organised, with only a small subpopulation of cells (cancer stem cells) having the capacity to self-renew.
The majority of tumour cells are differentiated non-tumour-initiating progeny. These cells may proliferate for a limited time but cannot maintain long-term tumour growth on their own.
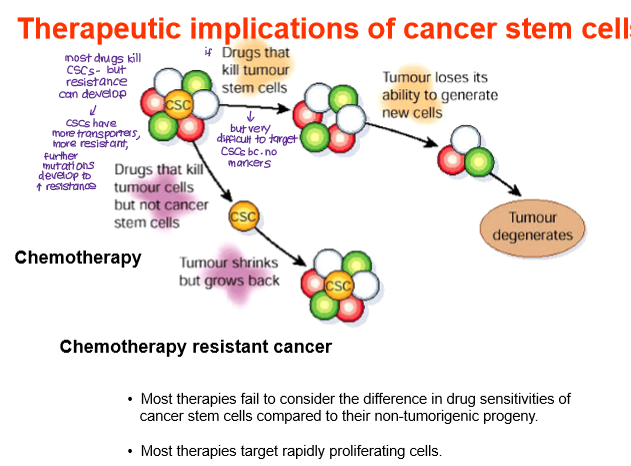
What are the therapeutic implications of CSCs?
Chemotherapy resistance
CSCs often have high drug efflux pump expression and resistance to apoptosis, develop mutations that increase resistance to drugs.
Most drugs will kill normal cancer cells but spare CSCs, which can self-renew and regenerate the tumor.