Chapter 6: Shapes of molecules and ions, electronegativity, polarity, intermolecular forces, hydrogen bonding
1/42
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
43 Terms
what is electron-pair repulsion theory?
electron pairs repel so that they are as far apart as possible → the arrangement of electrons minimises repulsion and therefore holds the bonded atoms in a definite shape
lines, wedges, and dotted wedges:

order of repulsion between bonded and lone pairs:

for every lone pair, a bond angle is reduced by…
2.5 degrees
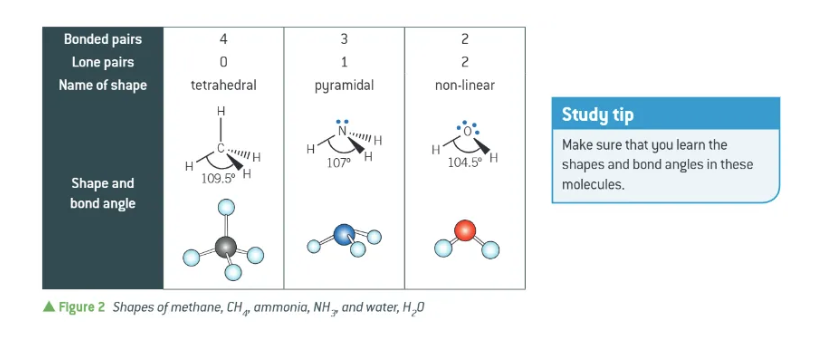
4 bonded pairs + 0 lone pairs =
3 bonded pairs + 1 lone pair =
3 bonded pairs + 2 lone pairs =
in electron-repulsion theory, multiple bonds are treated as…
the same as single bonds
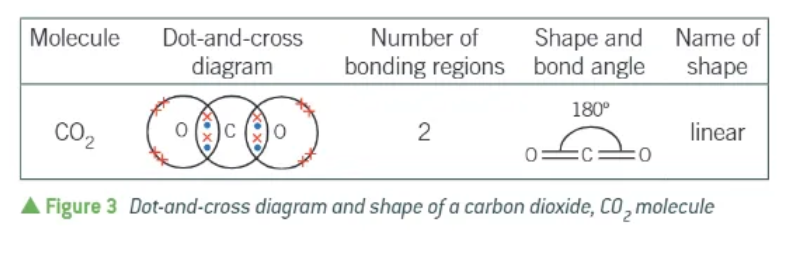
what shape does carbon dioxide have?
linear
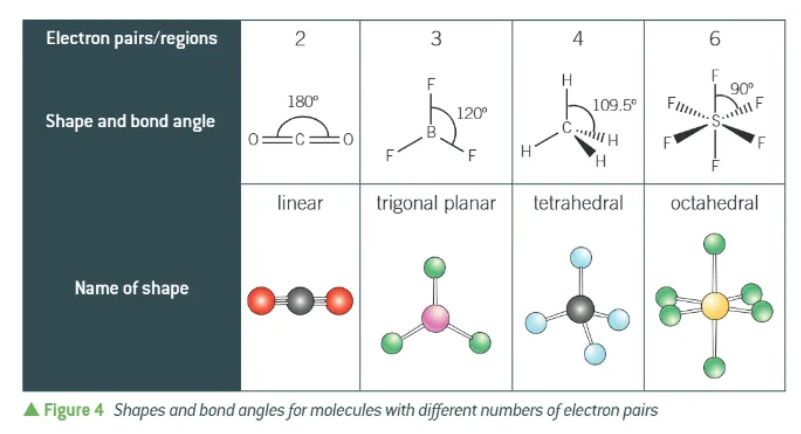
bond angles + names for 2,3,4, and 6 electron pairs + no lone pairs:
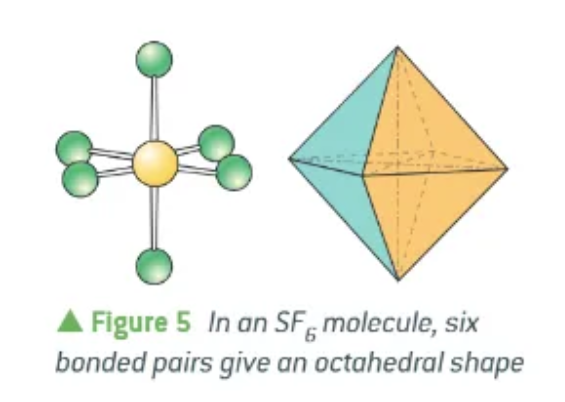
SF6 electron repulsion theory:
eight sides to the shape it makes
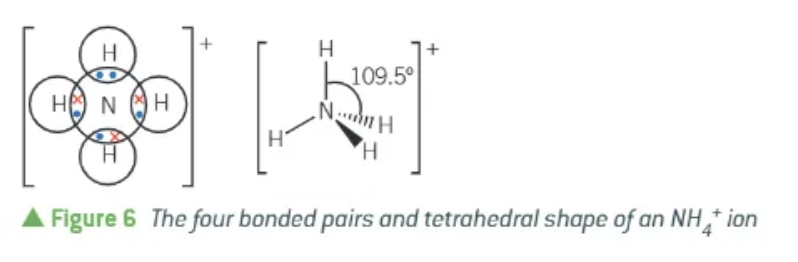
shape of ammonium ion:
tetrahedral
4 bonded regios
no lone pairs
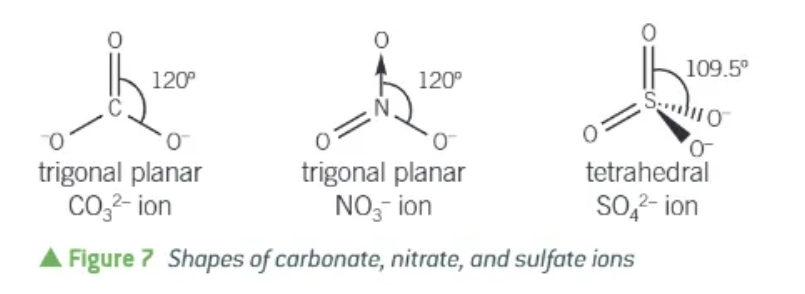
shapes of carbonate, nitrate, and sulphate ions:
electronegativity def.
the attraction of a bonded atom for the pair of electrons in a covalent bond
general patterns of electronegativity on the periodic table:
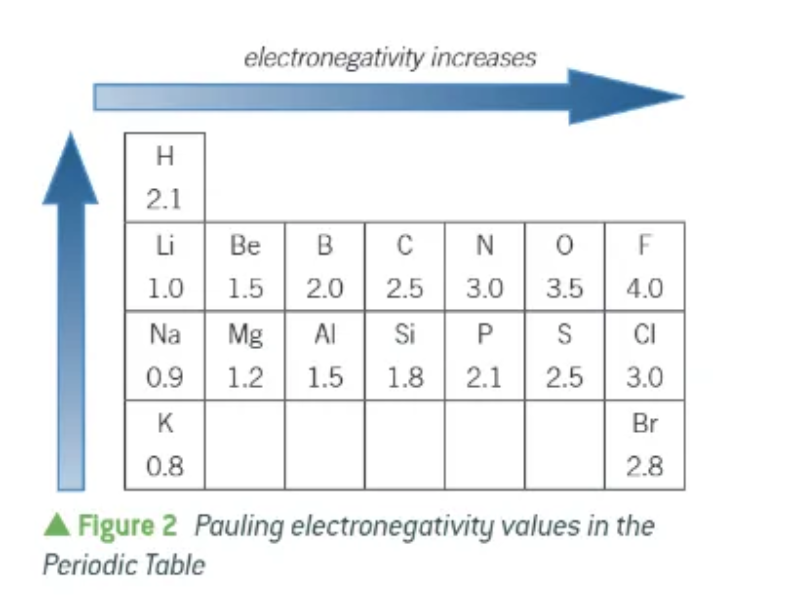
a large Pauling value indicates that the atoms of an elements are…
very electronegative
e.g. Fluorine is the most electronegative atom, and has a Pauling value of 4.0
how can we use the difference in electronegativities between 2 atoms to tell what kind of bond it will be?
0 difference = covalent
0 - 1.8 = polar covalent
greater than 1.8 difference = ionic
in a non-polar bond, the bonded electron will be…
shared equally between atoms
a bond will be non-polar when:
the bonded atoms are the same - e.g. F-F
the bonded atoms have the same/similar electronegativities - e.g. H-C
when is a bond polar?
the bonded atoms are different, e.g. H-Cl, and have different electronegativity values
→ e.g. Chlorine is most electronegative, and so the bonded electron pair is attracted closer towards the Cl atom .
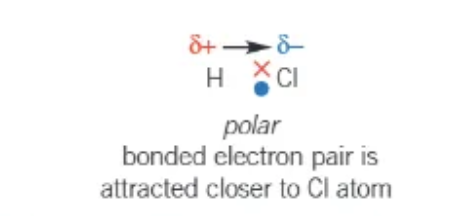
what does this mean in bond polarity → δ
small → this indicates a partial charge in a polar bond
def. of a dipole
the separation of opposite charges
if an atom has a larger electronegativity, which δ will it have?
δ- → the smaller will have a δ+
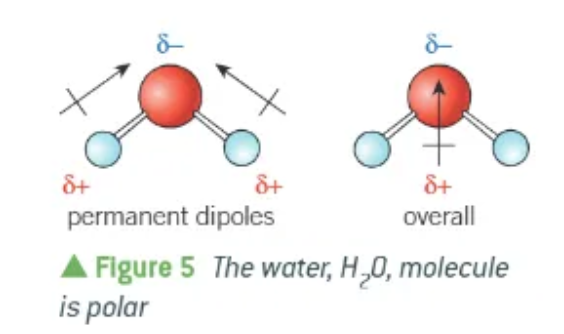
why is a water molecule polar?
the 2 O-H bonds are permanent dipoles
the 2 dipoles act in different directions (but not directly opposite!)
overall, the oxygen end of the molecules has a δ- charge, and the hydrogen has a δ+ charge.
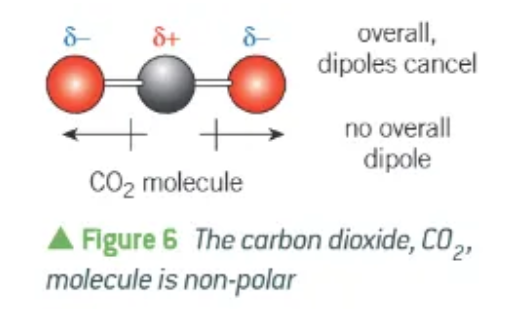
why is a CO2 molecule non-polar?
both C=O bonds are polar, and have permanent dipoles
the 2 dipoles act in opposite directions, exactly opposing each other
the whole molecule + dipoles cancel out → overall dipole = 0
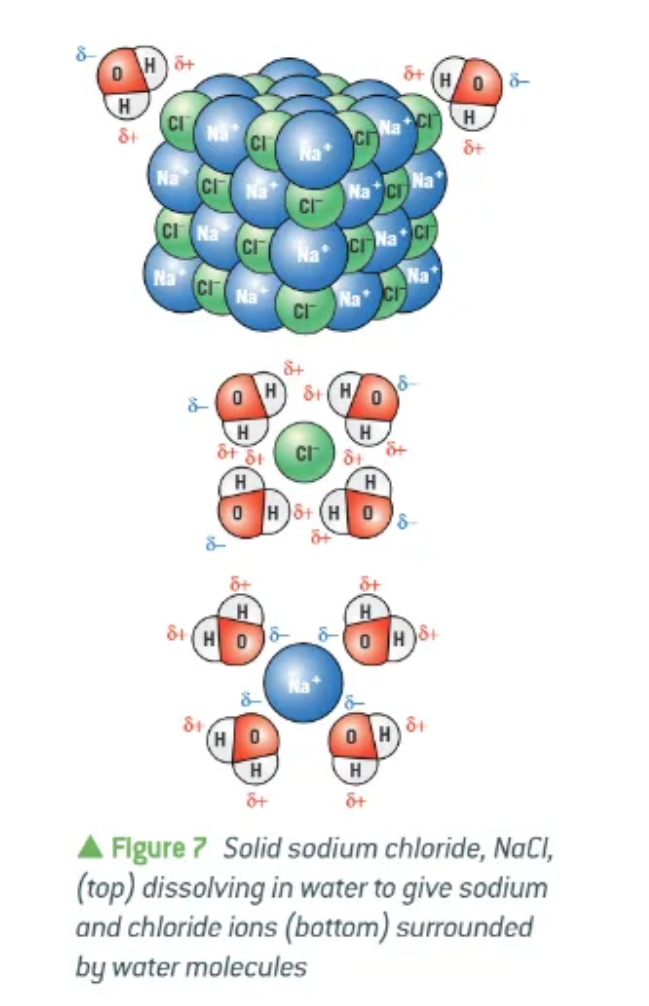
polar solvents + polar solutes:
positive ions are attracted to the δ- of the water
negative ions are attracted to the δ+ of the water
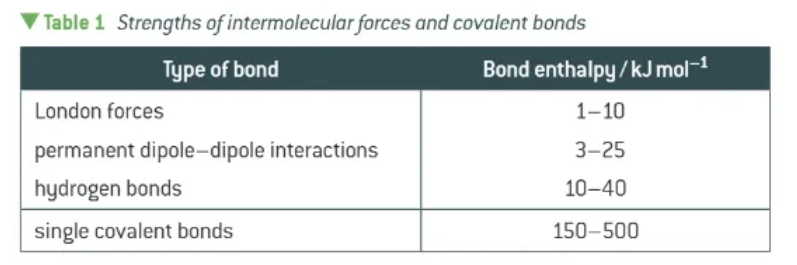
compare the strengths of London forces, permanent dipole-dipole interactions, Hydrogen bonds, and single covalent bonds:
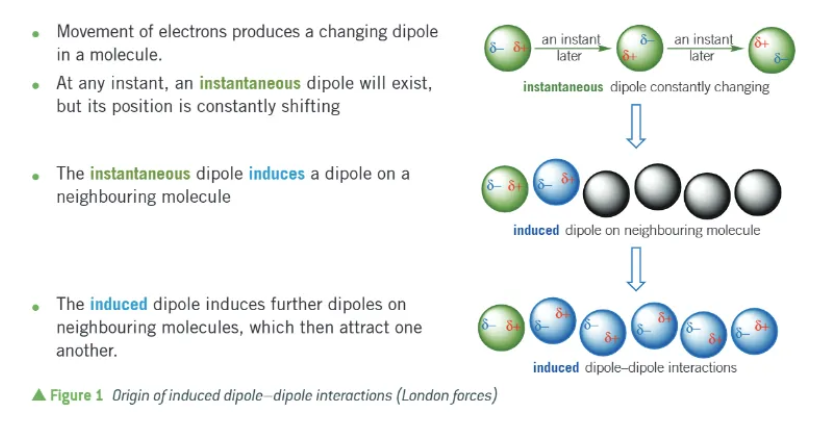
what are London forces?
the forces that act between instantaneous dipoles:
movement of electrons produces a changing dipole in a molecule
at any instant, an instantaneous dipole will exist, but its position is constantly shifting.
the instantaneous dipole induces a dipole on a neighbouring molecule
the induced dipole induces further dipoles on the neighbouring molecules, which then attract one another.
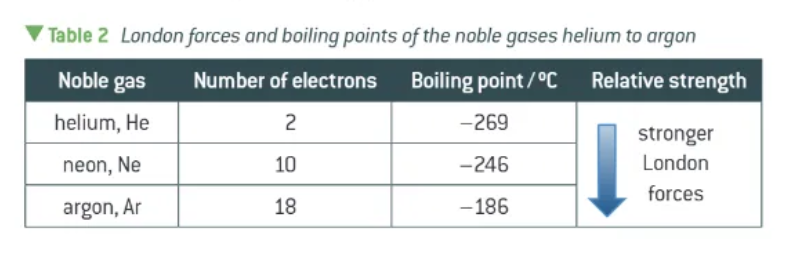
how does the strength of London forces vary between different elements/molecules?
more electrons = larger instantaneous + induce dipoles
greater the induced dipole-dipole interactions
stronger attractive forces between molecules
this affects boiling point !!
permanent dipole-dipole interactions:
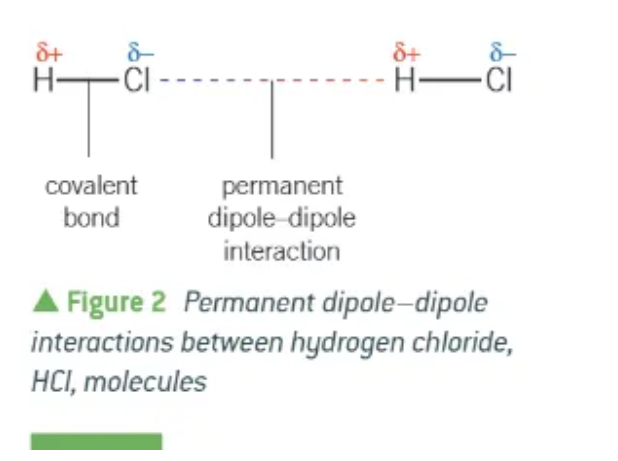
F-F and H-Cl have the same number of electrons + the same shape. The strength of their London forces should be very similar. Why is the boiling point of H-Cl so much higher than F-F?
The permanent dipole-dipole interactions
H-Cl = polar molecule
extra energy is needed to break these additional pd pd bonds
what do substances with pd pd forces also have?
london forces!!
what is a simple molecular substance?
made up of simple molecules → small units containing a definite number of atoms with a definite molecular formula → e.g. H2O
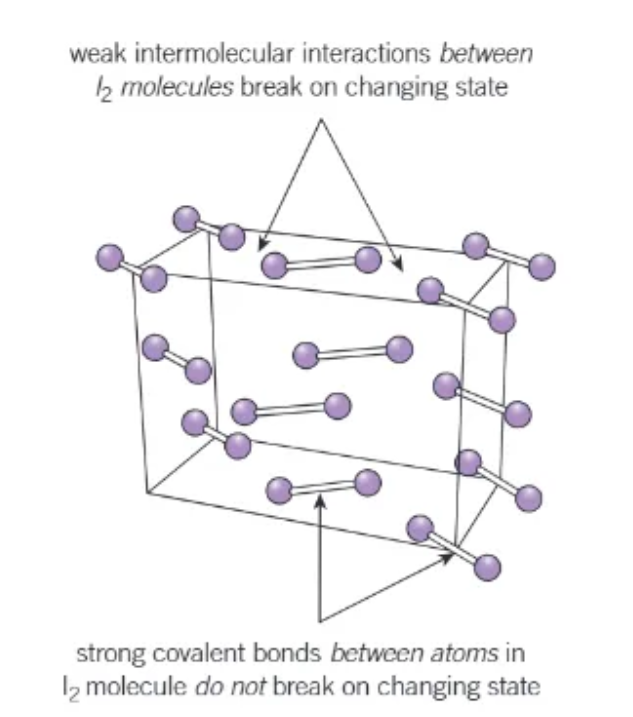
forces in a simple molecular lattice:
the molecules are held in place by weak intermolecular forces
the atoms within each molecules are bonded together strongly by covalent bonds.
boiling points of simple molecular substances:
low melting + boiling point:
only the weak intermolecular forces break
the covalent bonds are strong and do NOT break
solubility of non polar and polar substances:
non-polar = soluble in non-polar solvents
polar = soluble in polar solvents
solubility of non-polar simple molecular substances
insoluble in polar solvents → i. bonding in polar solvent is too strong to be broken
soluble in non-polar solvents → intermolecular forces form between the molecules and the solvent → interactions weaken the intermolecular forces in the simple molecular lattice → i.forces break + compound dissolves
some compounds, like ethanol, that contain non-polar and polar parts can…
dissolve in non-polar and polar solvents
hydrophilic part (e.g. O-H) interacts with water
hydrophobic part (carbon chain) will not
can simple molecular structures conduct electricity?
no → no charged particles that can move
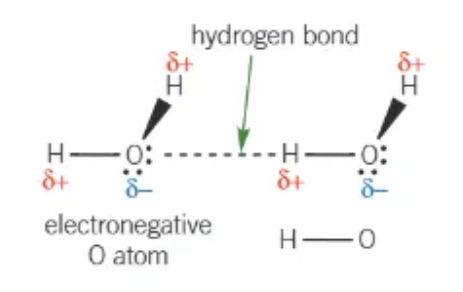
which 4 elements can be in a hydrogen bond?
F, H, O, N
hydrogen bond occurs between…
a lone pair + a hydrogen atom
draw a hydrogen bond between 2 water molecules:
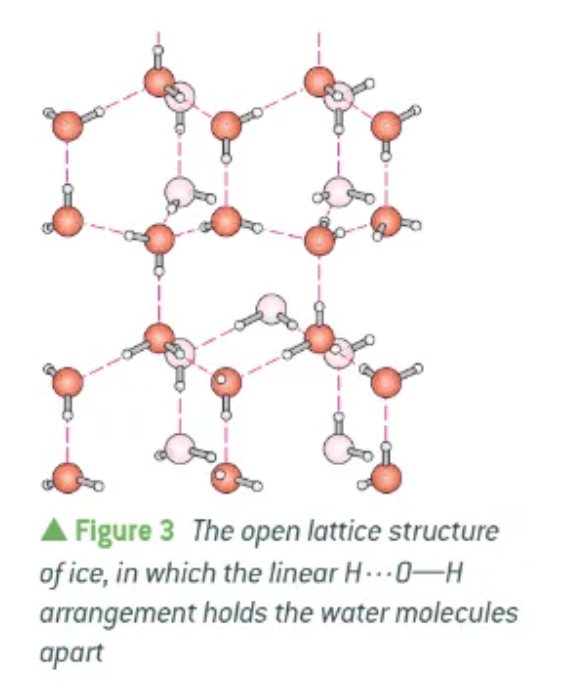
why is ice less dense than water?
Hydrogen bonds hold water molecules apart in an open lattice structure
The water molecules in ice are held further apart than in water.
Solid ice is less dense than liquid water and floats.
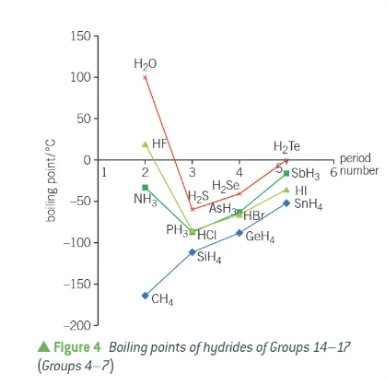
why does water have an unusually high melting point?
the hydrogen bonds!!
other unusual properties of water:
relatively high surface tension
high viscosity