Potential Energy Graphs and Reaction Shifting
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
potential energy reactants
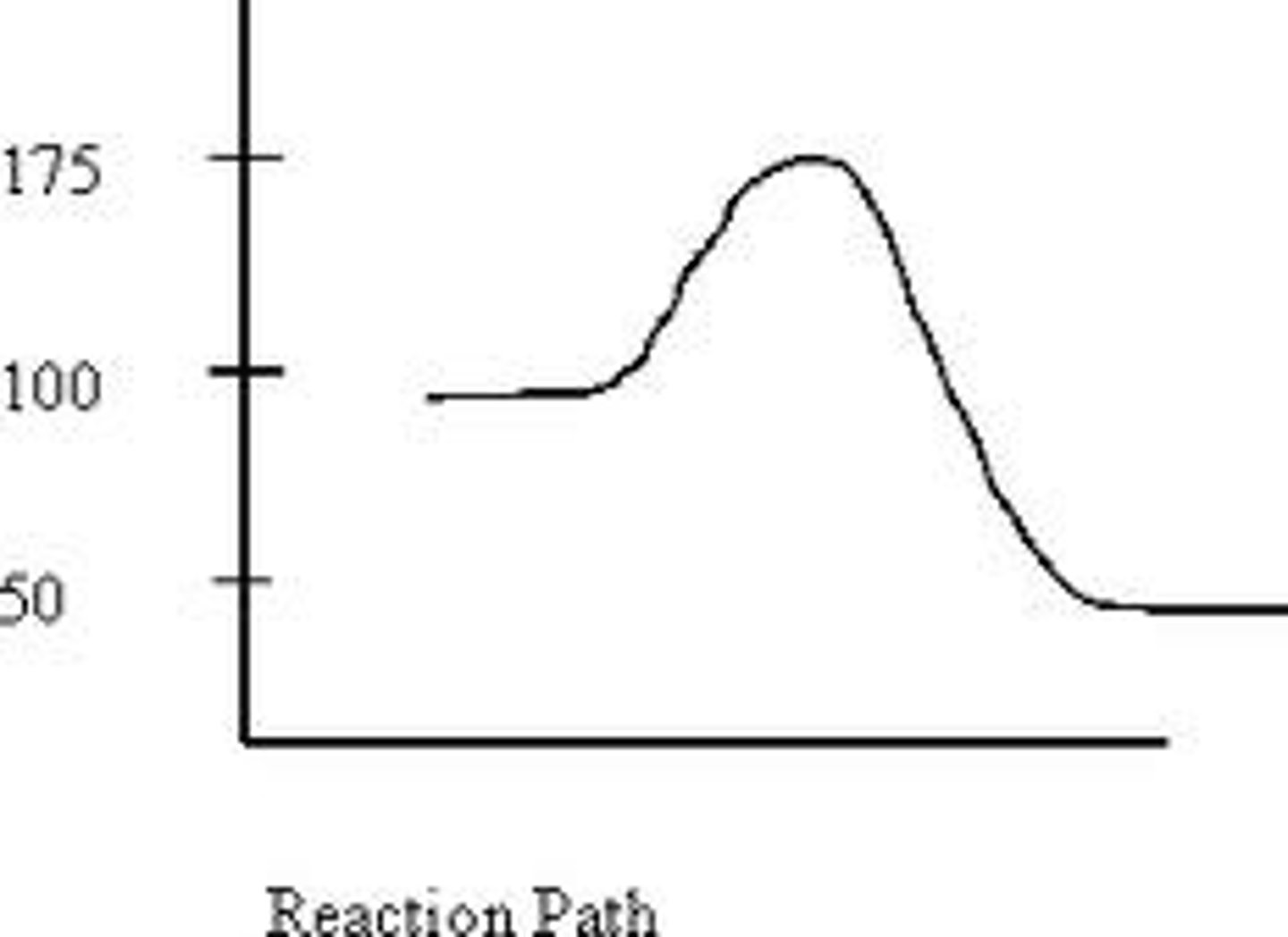
What A represents on the diagram in the forward direction
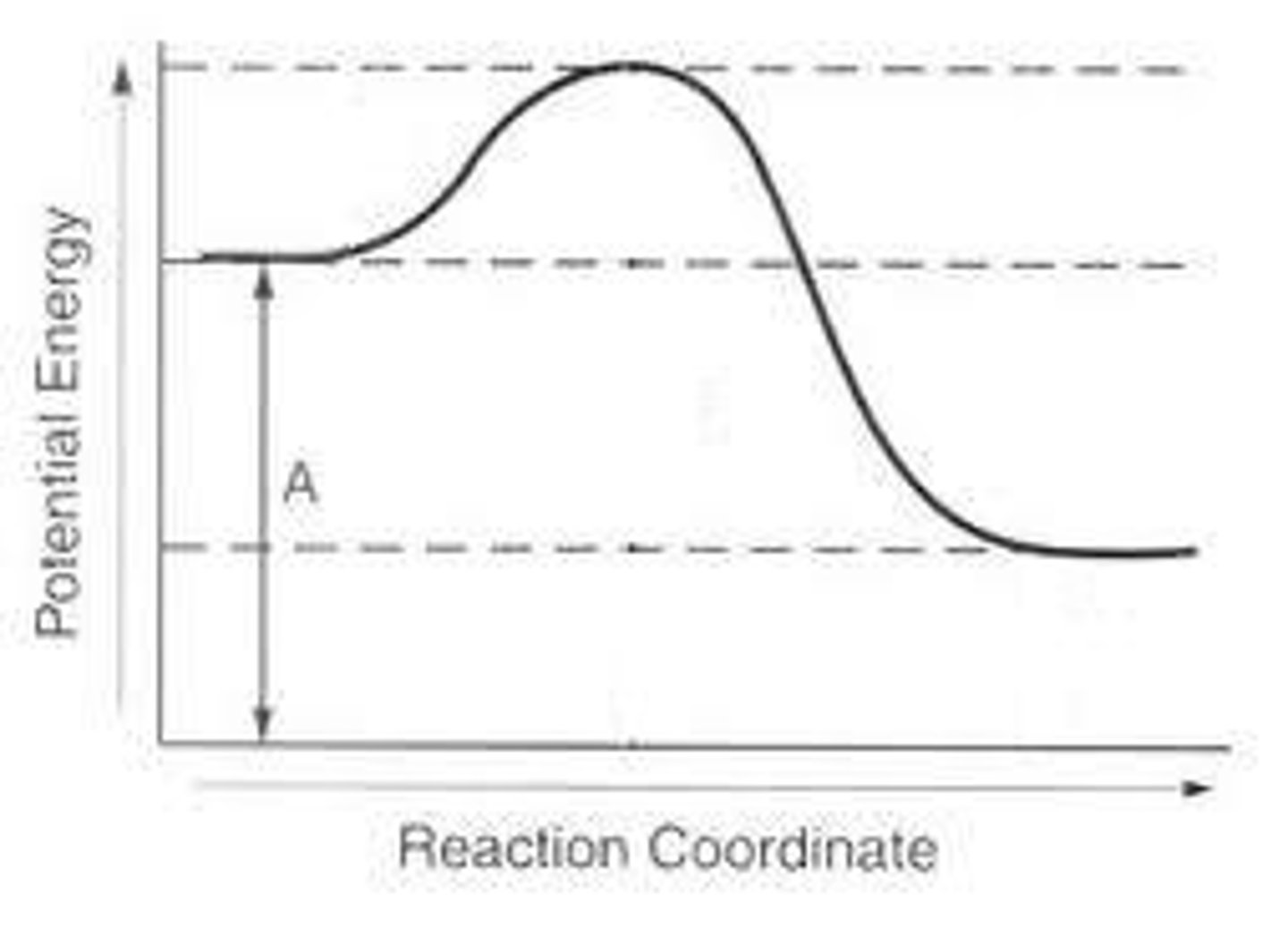
= Top - Reactants
The Calculation for the Activation Energy
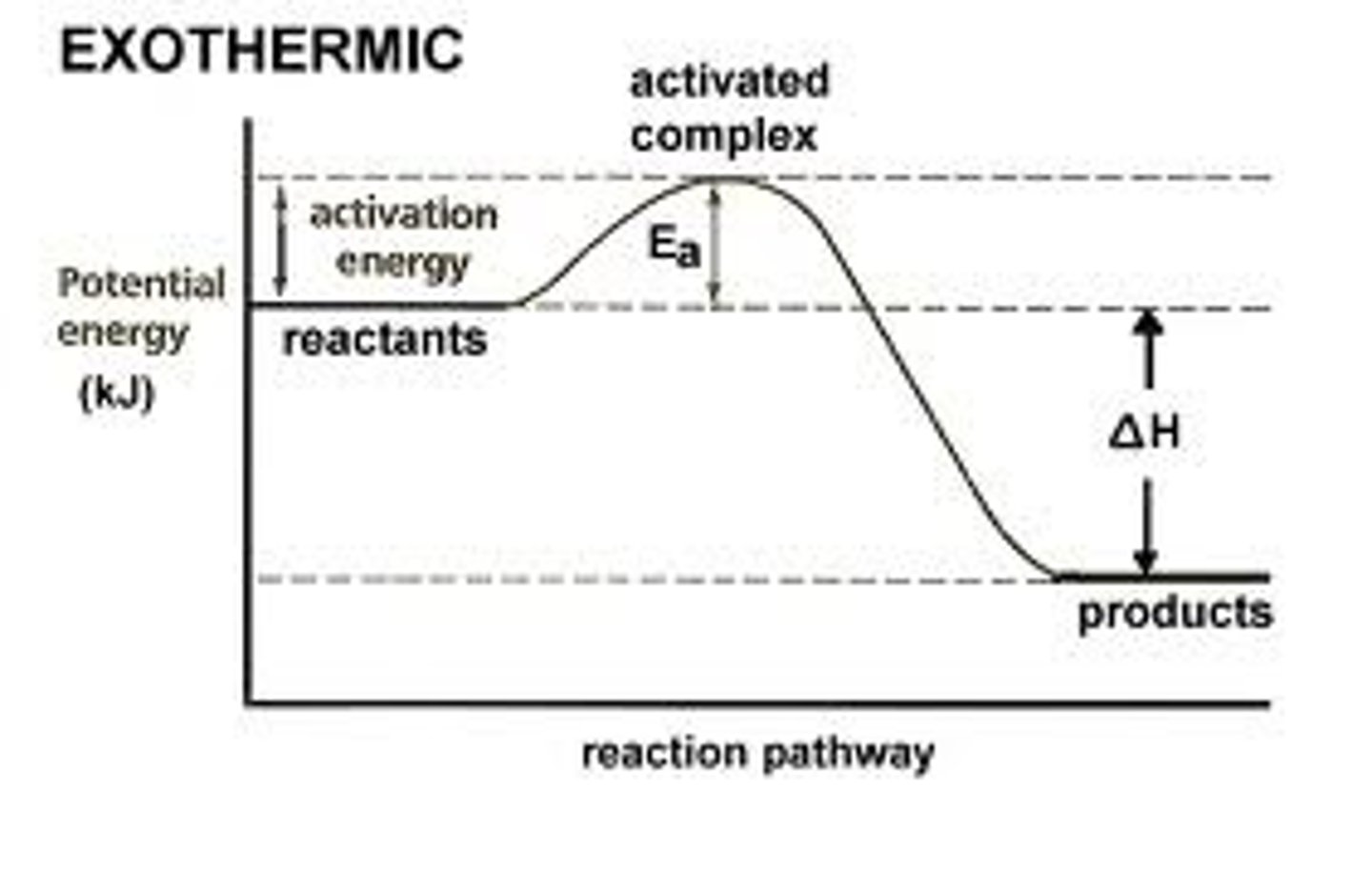
= Products - Reactants
Calculation for the Enthalpy (deltaH)
Exothermic
Negative change in energy is a __ reaction
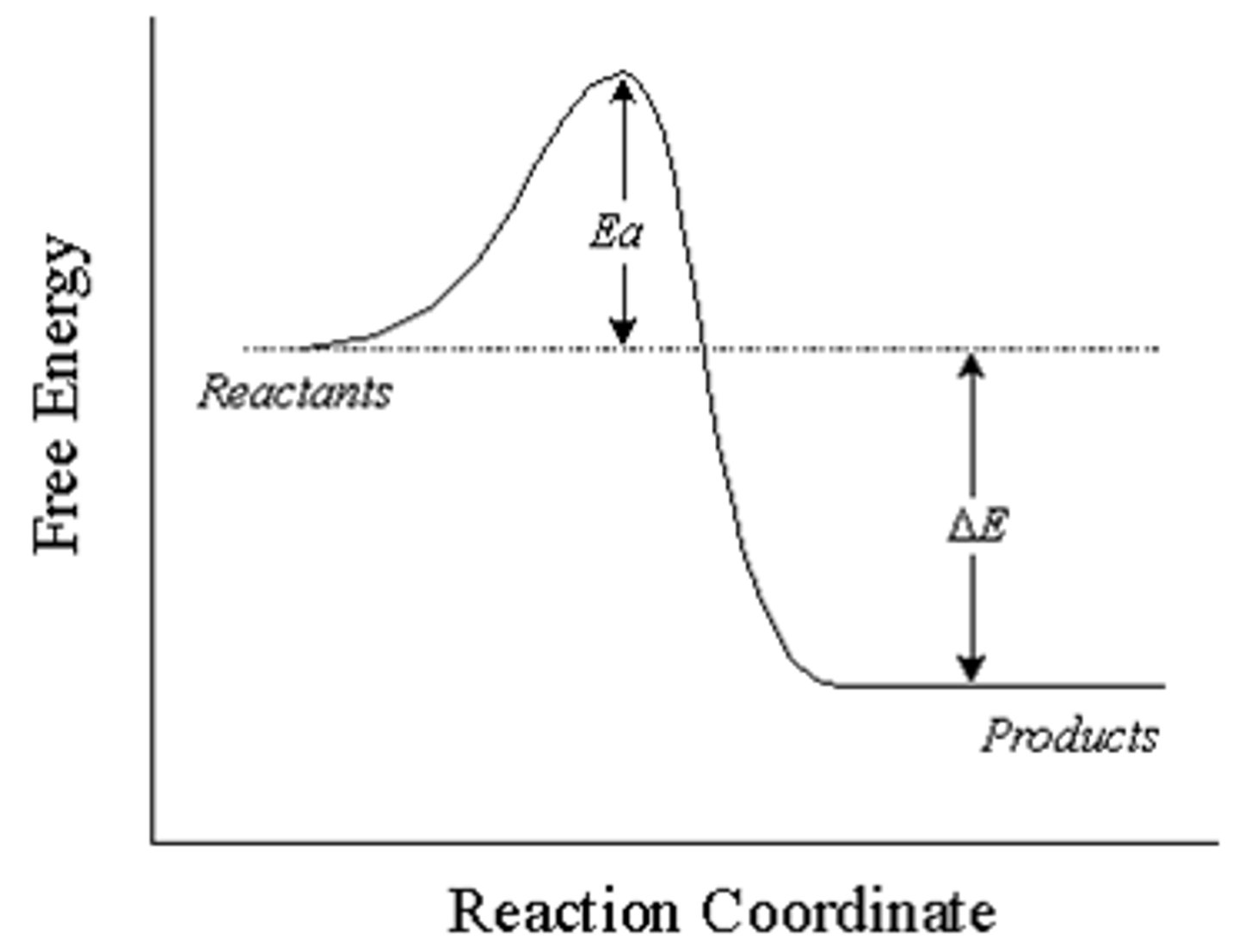
Endothermic
Positive change in energy is a __ reaction
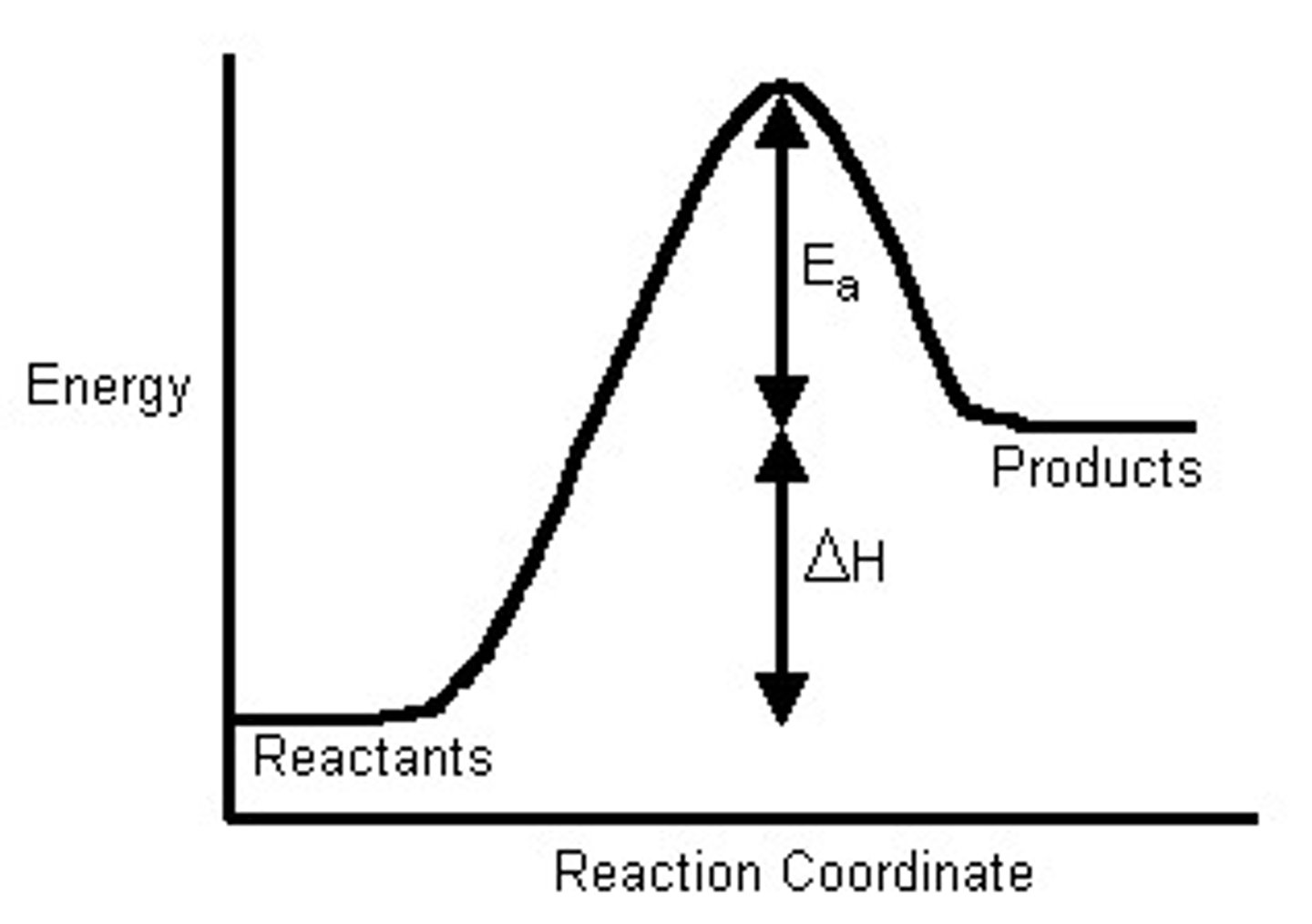
-50kJ
The delta H of the forward direction of this reaction
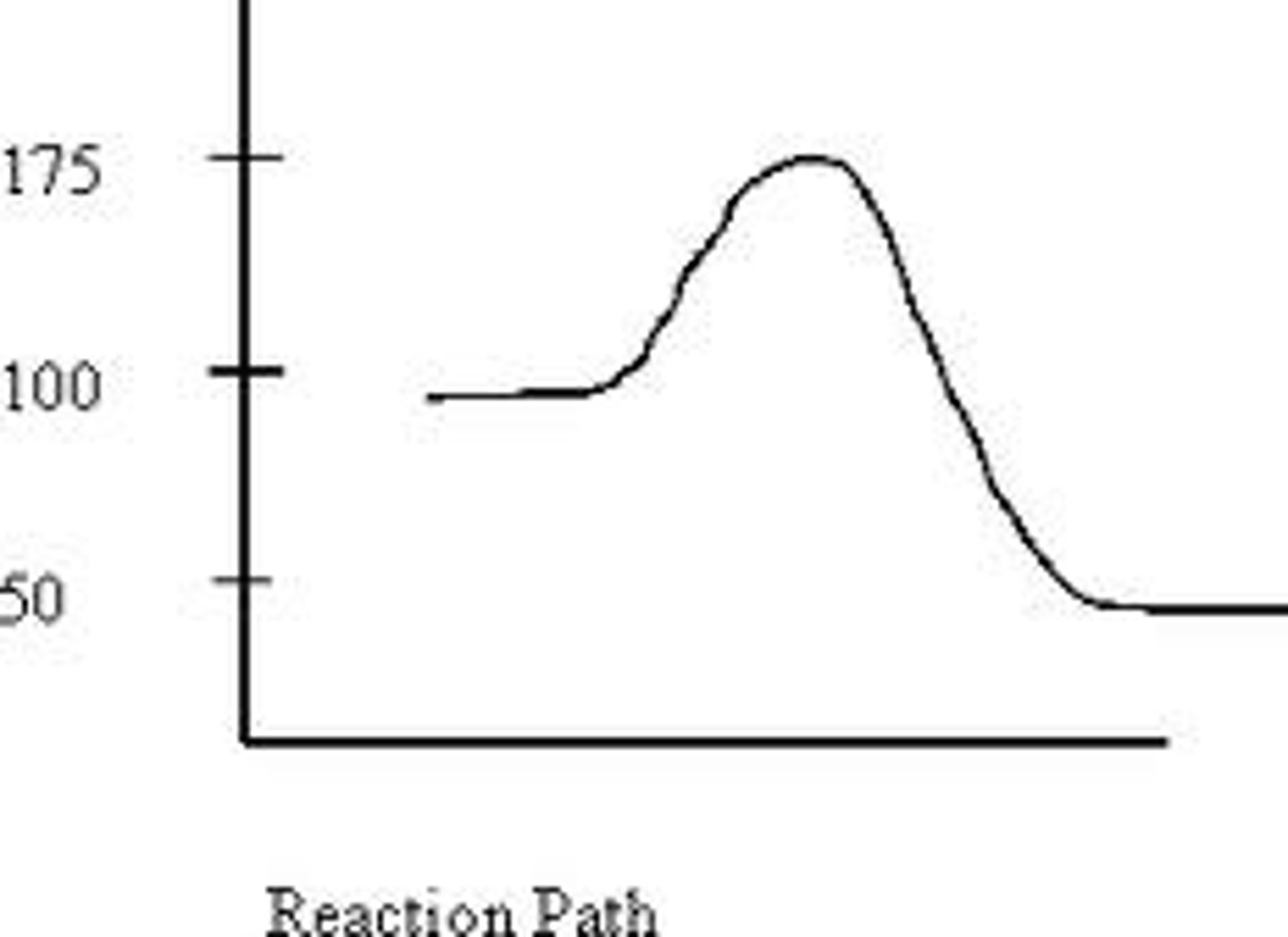
+50kJ
The delta H of the reverse direction
shift left
Add more carbon dioxide
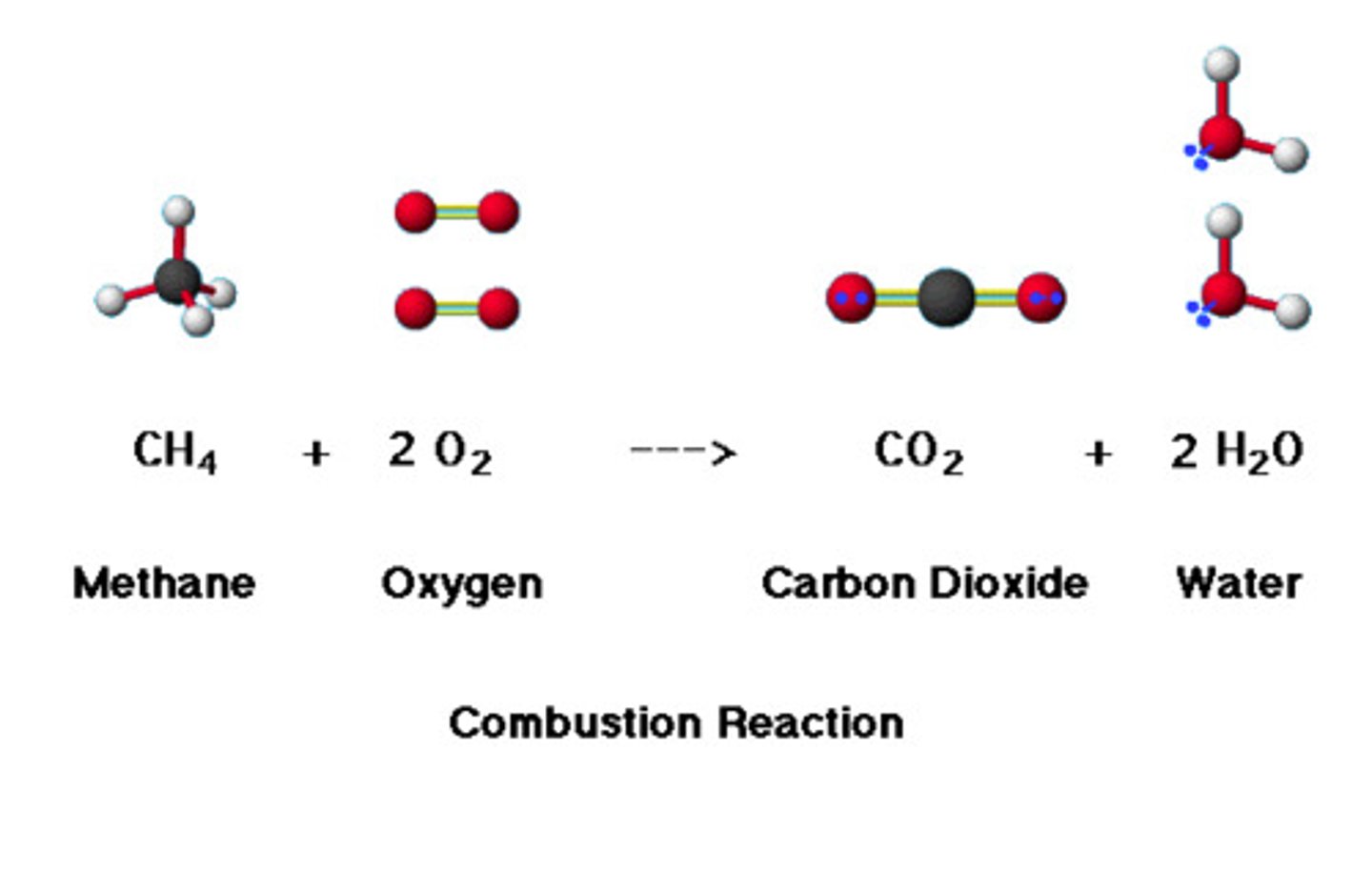
shift right
Remove carbon dioxide
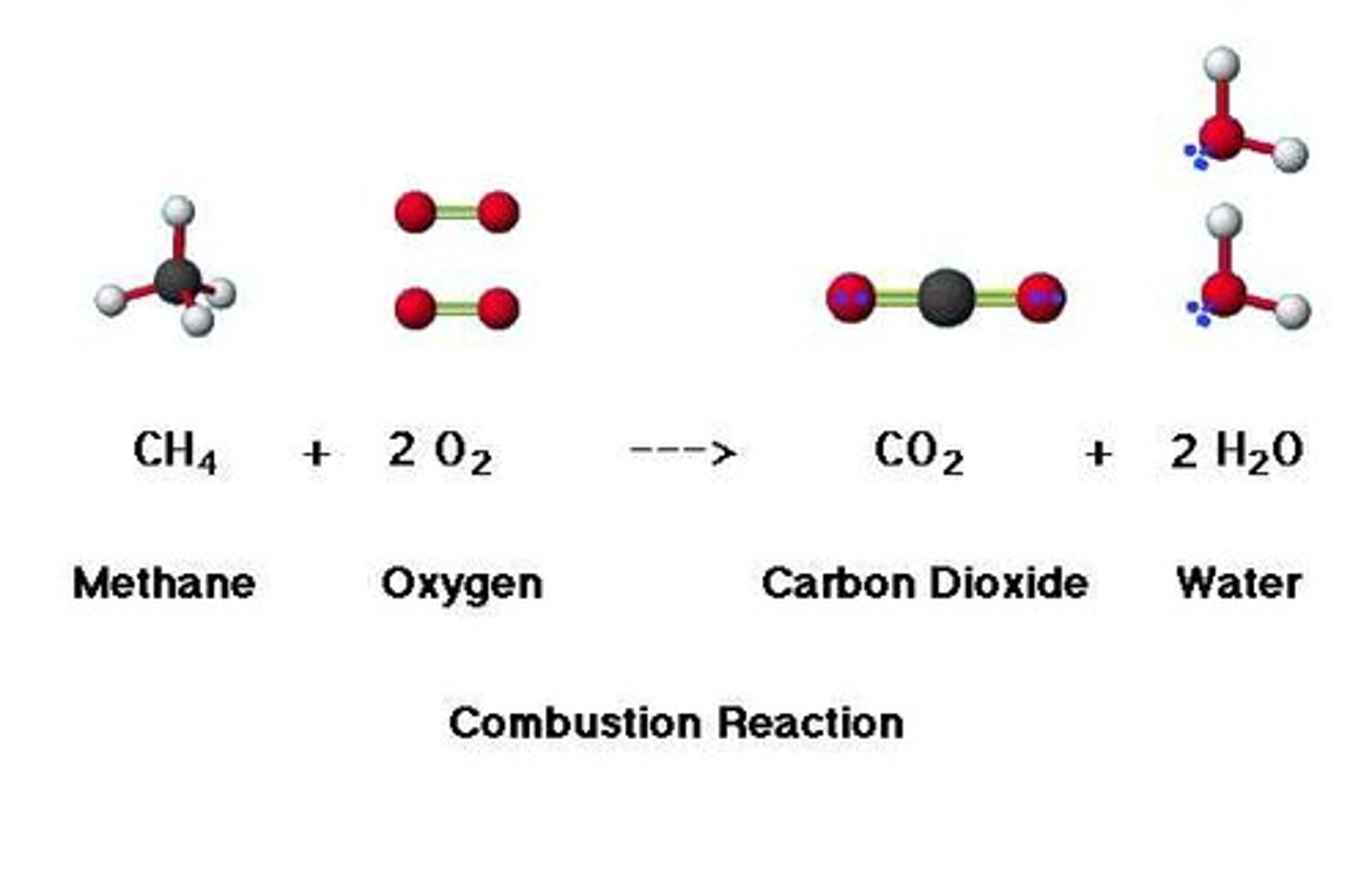
no shift
Increase the pressure
Collision Theory
Particles must collide with: reactant, enough Energy, correct orientation,
Reaction Kinetics
1) increase surface area, 2) increase pressure/decrease volume, 3) add a catalyst, 4) increase concentration, 5) increase energy/temp