oxygen binding proteins
1/20
Earn XP
Description and Tags
week 2 i cant believe i went to this workshop twice
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms
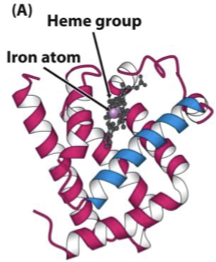
myoglobin (Mb)
single polypeptide chain of globular proteins, tertiary structure
O2 storage (primarily muscle tissue)
Contains one haeme prosthetic group, binds one O2 molecule - high affinity
two redox states of iron
Fe2+ (ferrous)
can bind to O2
toxic to cells
Fe3+ (ferric)
cannot bind to O2
Fe3+-bound Hb compromises the body’s ability for O2 transport and storage
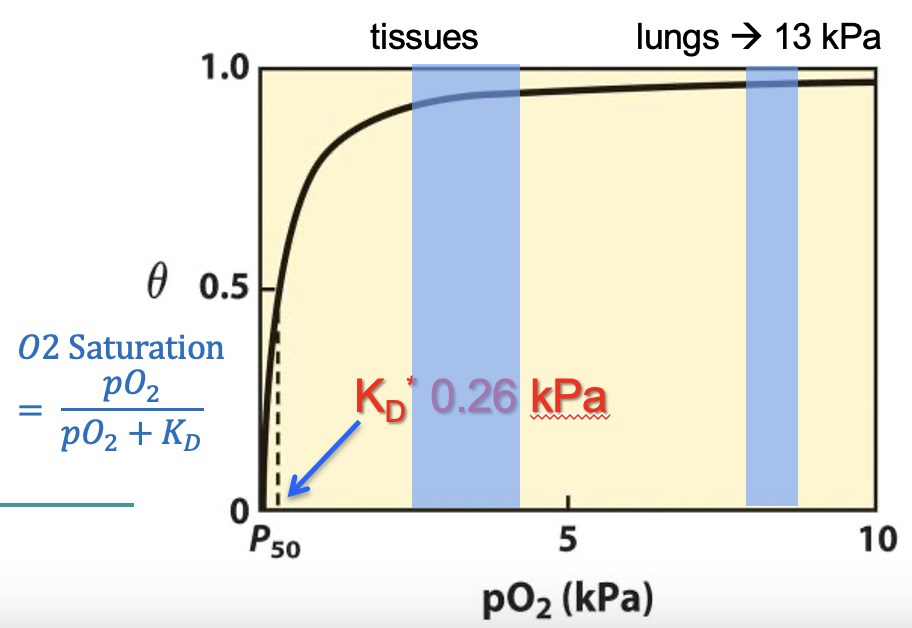
O2 dissociation curve
graph showing the relationship between the amount of O2 (saturation) bound to Mb or Hb vs pO2
O2 dissociation curve for myoglobin
Mb is almost completely saturated at high and low pO2
Very similar in the lungs and in the tissue
Hyperbolic curve
This is great for a STORAGE protein!
myoglobin KD
KD = dissociation constant
measure of affinity of a protein to its ligand → high affinity = low KD
Mg has a low KD (loves to bind hates to dissociate)
pO2 when 50% of binding sites are bound to O2 = KD
Haemoglobin (Hb)
Has 2 identical alpha subunits and 2 identical beta subunits (a2ß2) globins
“alpha globin chain”: each globin is one individual protein making up the quaternary complex
1 haeme and 1 Fe2+ subunit
Hb binds up to 4 O2 molecules
better at transporting O2
sensitive to small changes in pO2
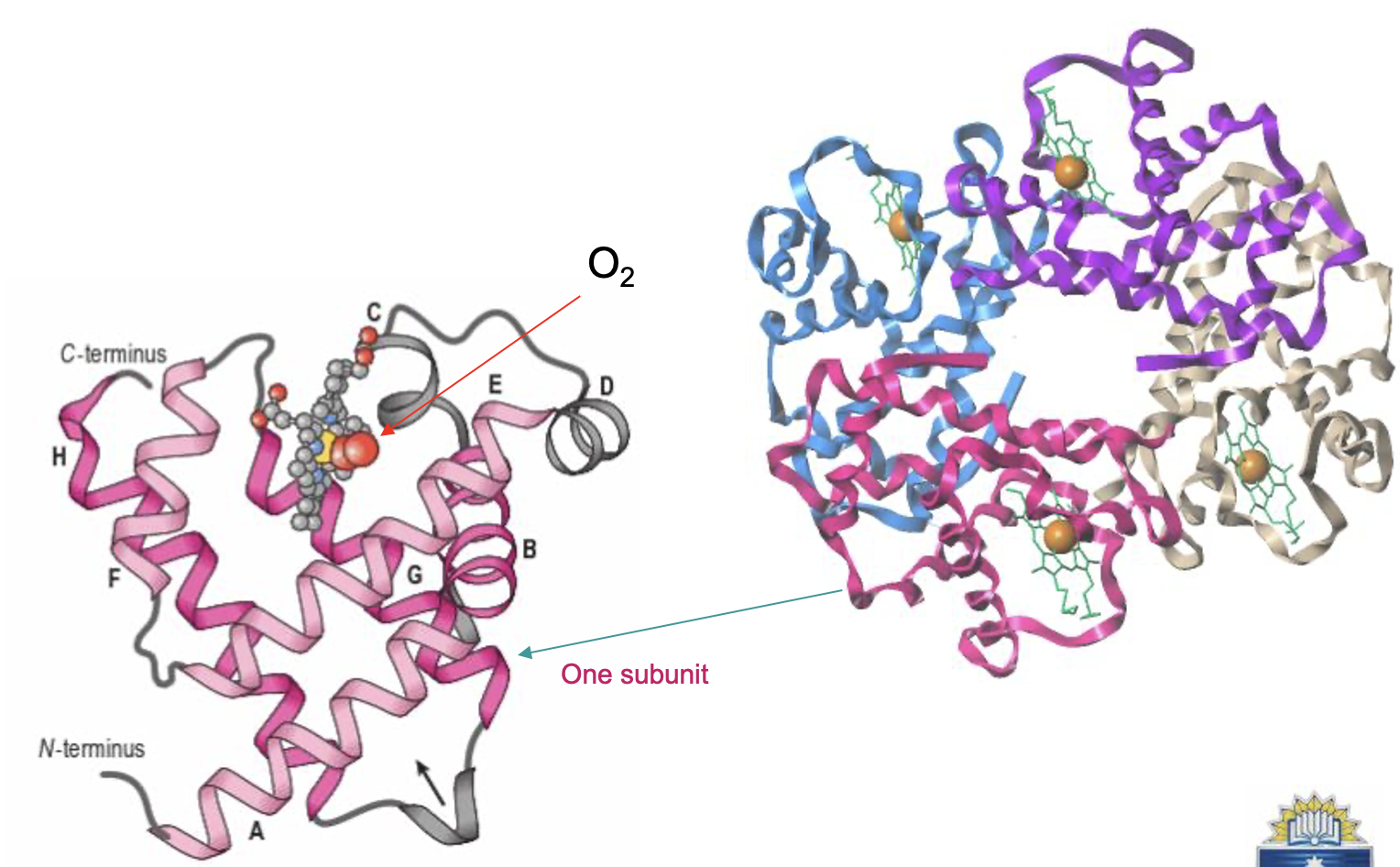
structure of haeme
porphyrin ring covalently bound in a deep pocket in each Hb subunit (protect the Fe2+!)
Fe2+ ion in centre
Fe2+ makes 6 covalent bonds
4 to the planar haeme ring
one to a Histidine amino acid of Hb
one to O2
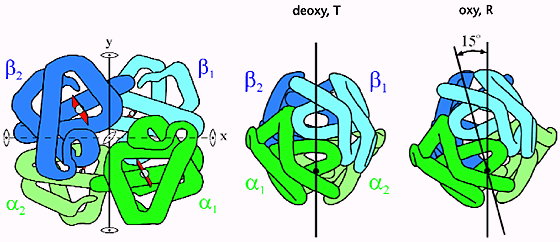
deoxygenated (Tense) state:
concave shape
high affinity for oxygen
oxygenated (Relaxed) state
flat, planar shape
low affinity for oxygen
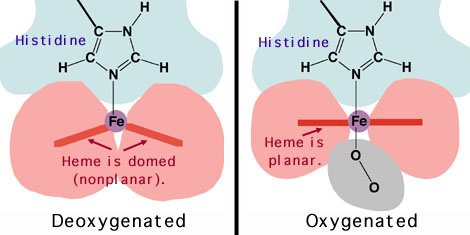
haemoglobin colour (deoxygenated vs oxygenated)
deoxygenated (Tense) state: darker red
oxygenated (Relaxed) state: lighter red (scarlet)
Pulse oximeter uses colour to detect pressure of oxygen by UV light
cooperative binding
Hb can change its affinity for O2 from high affinity (in the lungs) to low affinity (at the tissues)
affinity dependent on partial pressure of O2
Once 2 subunits, in the T state, bind O2 a T → R transition occurs
α and β subunits slide past each other, breaking ionic bonds that stabilize the T-state
makes it easier for the remaining subunits to bind O2
O2 dissociation curve for haemoglobin
S shaped curve due to changing affinity
dissociation high in tissues low in lungs
allosteric effectors
Haemoglobin can bind other small molecules at sites away from the O2-binding site
H+ (increased acid load)
CO2 (increased acid load) (forms carbaminoHb)
2,3-bisphosphoglycerate (2,3-BPG) (First step of glycolysis: form 2,3 BPG)
binding to allosteric site
→ conformational change in Hb structure
→ decreased affinity for O2 and altered functionality
→ T-state (deoxy) stabilises (is favoured)
→ off-loads more O2
factors allowing Hb to remain relaxed in lungs despite when pO2 levels drop
Other factors aid in Hb's changing affinity
pH (H+, CO2)
temp
2,3-BPG
Therefore Hb can remain highly saturated even with large decreases in pO2
Lungs have low temp, high pH, high 2,3-BPG
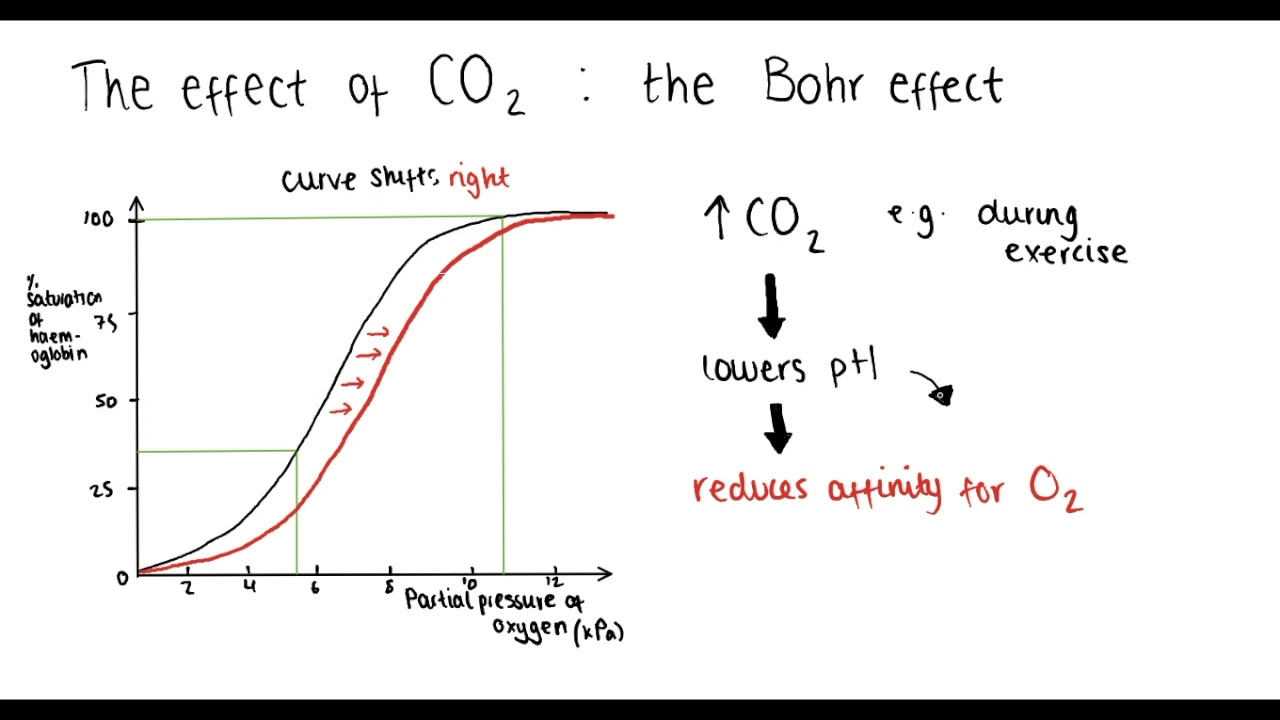
the BOHR effect
affinity of hemoglobin for oxygen decreases when the pH of the blood decreases
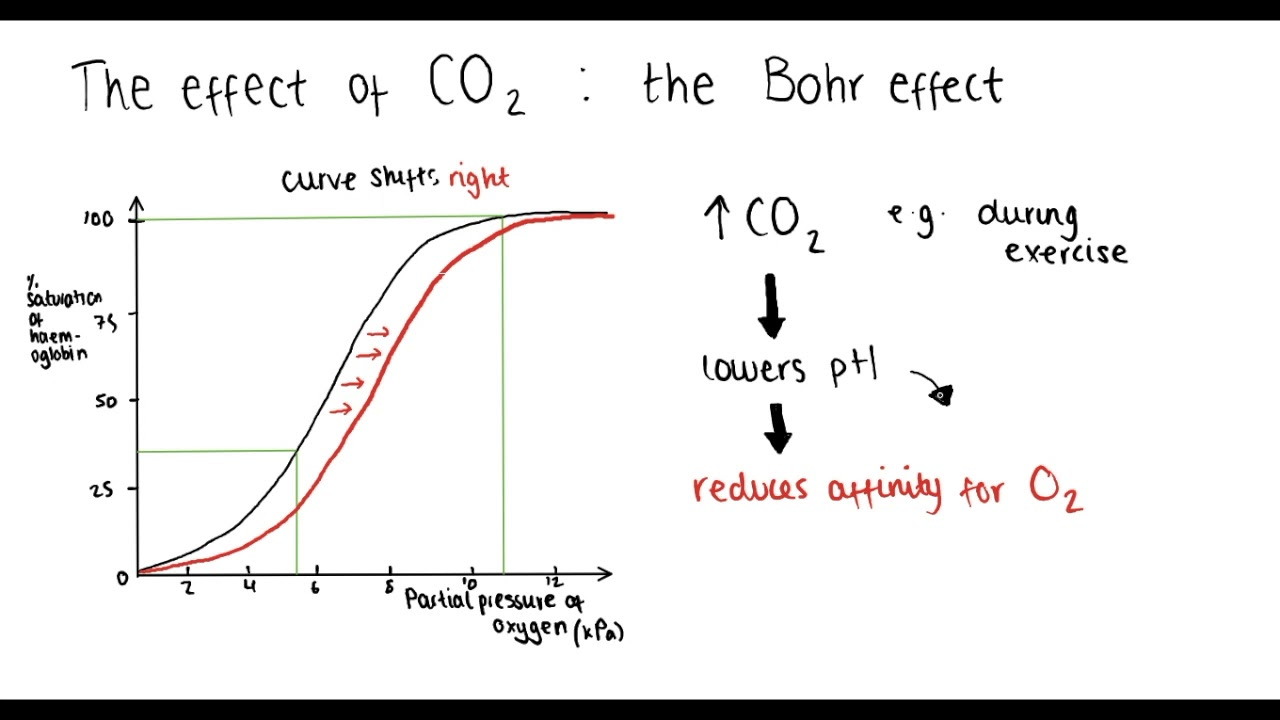
↓ H+ (increasing pH): BOHR effect
occurs in the lungs
T→R transition → Hb releases H+
R state stabilises → picks up O2
Causes a shift to the left of the ODC
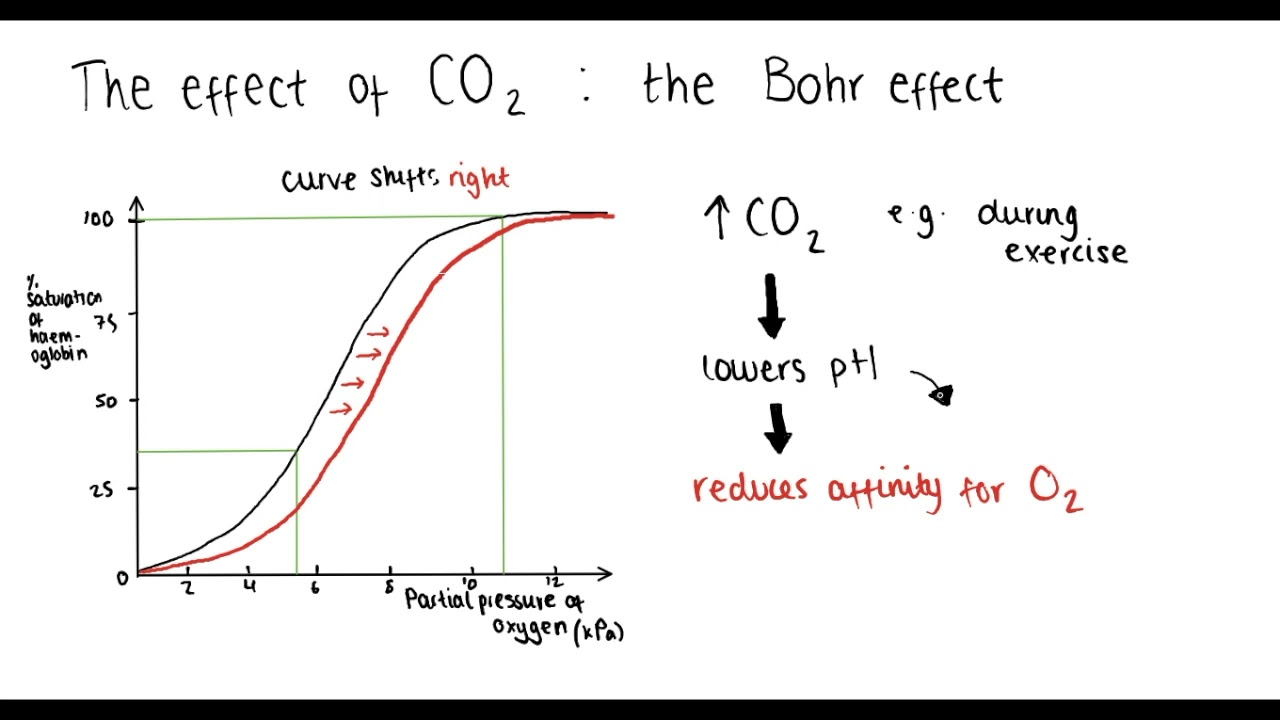
↑ H+ (lower pH): BOHR Effect
occurs at the periphery
Hb binds H+ (acts as buffer)
T-state stabilises → off-loads O2 to periphery.
Causes a shift to the right of the ODC
(e.g. exercise)
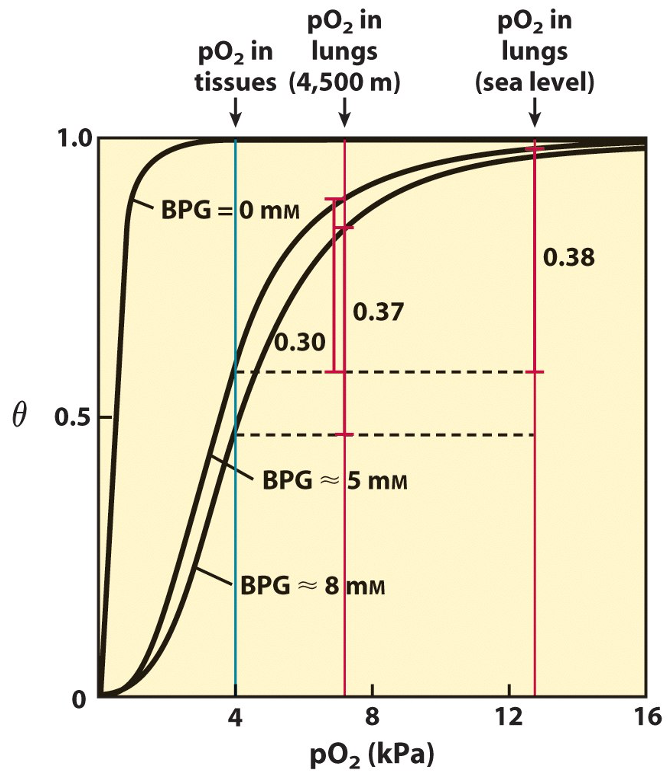
2,3-BPG regulation of O2 off-loading
Binding of 2,3-BPG dramatically decreases Hb affinity for O2
2,3-BPG ↑ at high pressures where levels of O2 ↓ (high altitudes (during acclimatisation))
shifts ODC curve right
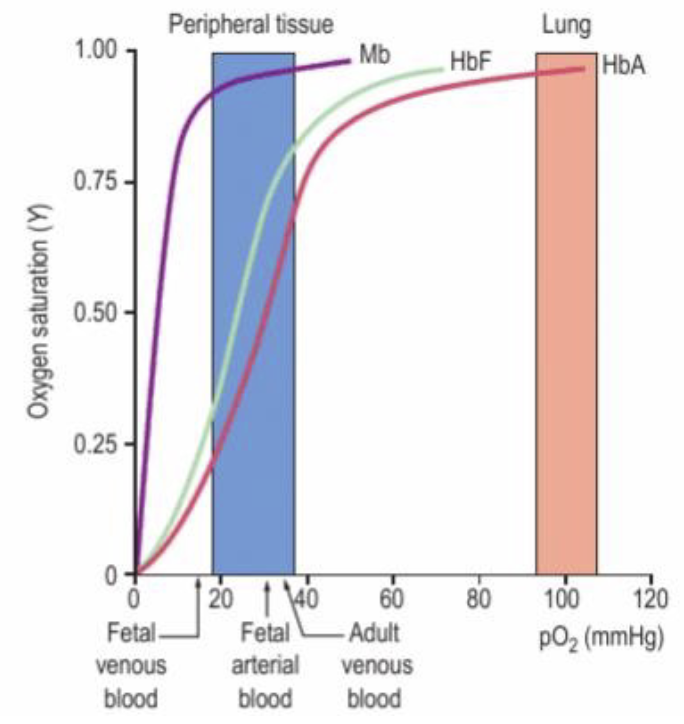
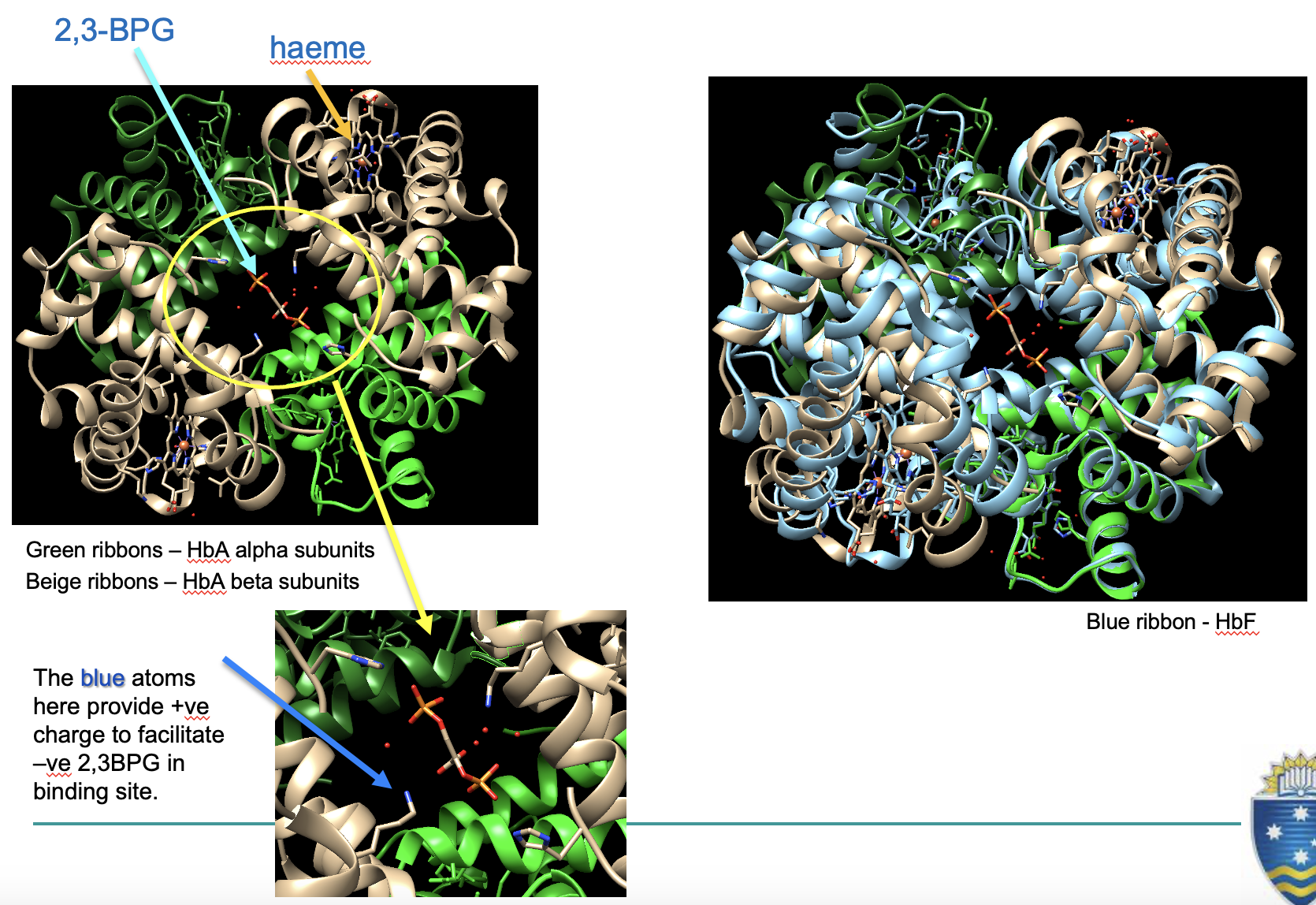
foetal haemoglobin (HbF)
the foetus depends on mother for oxygen, so their Hb has higher affinity (can bind oxygen faster)
HbF contains 2 γ subunits instead of ß subunits (α2γ2)
2,3-BPG cannot bind as well, increasing O2 affinity
(due to lack of positive amino acid to bind to)
lose HbF across foetal development
carbon monoxide poisoning
Binds to Fe2+ in haeme with 200x affinity than O2
Bound CO prevents O2 from binding
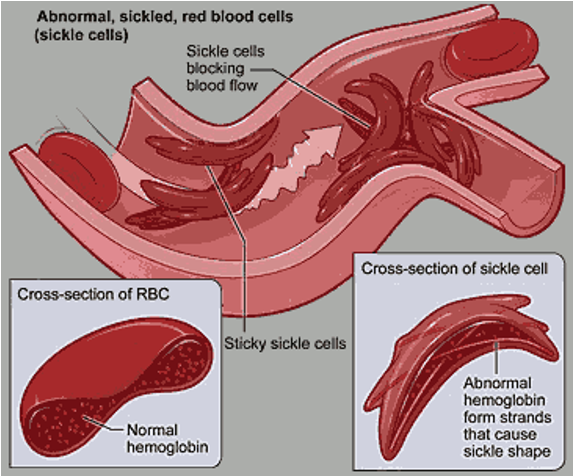
sickle cell anaemia (HbS)
Sickle shaped RBCs caused by a Hb mutation
Mutation of glutamate (a charged, polar amino acid) for a non-polar valine
HbS is insoluble
RBCs cannot go through blood vessels and get stuck → pain
Cannot carry oxygen efficiently → hypoxia
killed by spleen bc low haematocrit → anaemia
Hb blood pressure regulation
Nitric oxide (NO) is produced in the tissues
when unbound: vasodilates the walls of cardiac blood vessels (BP↓)
Hb binds to NO
NO binds to specific cysteine residues in Hb and to the Fe2+ in haeme
Excess haemoglobin/RBCs:
vasoconstriction → hypertension