as level practicals
1/5
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
6 Terms
making up a volumetric solution
weight out the solid onto a weighing boat
add the solid to a beaker
reweigh the weighing boat and record the difference in mass
add 100cm³ of distilled water to the beaker (use some to wash out the weighing boat) and use a stirring rod to help to dissolve some of the solid
pour into a 250cm³ volumetric flask
rinse beaker and funnel and add washings to a volumetric flask
make up to the line with the meniscus at the bottom of line using a dropping pipette for the last few drops
invert several times to ensure a uniform solution
carrying out an acid base titration
rinse the equipment (burette with acid, pipette with alkali, conical flask with water)
pipette 25cm² of alkali into conical flask
touch surface of alkali with pipette
make sure the jet space in the burette is filled with water
add a few drops of indicator to the alkali
add a white tile underneath the flask to help observe the colour change
add acid to the alkali while swirling the mixture and add the acid drop by drop at the end
note burette reading before and after adding the acid
repeat titration until at least 2 concordant results are obtained
measuring enthalpy change of reaction (solution)
wash equipment with solutions to be used
dry cup after wash
put polystyrene cup in beaker
measure out desired volumes of solutions with volumetric pipettes and transfer to insulated cups
clamp thermometer in place
record initial temperature of solutions
add second reagent to cup
stir mixture
record temperature every minute for several minutes
draw a cooling curve
purifying an organic liquid
pour the distillate of an impure product into a separating funnel
add sodium hydrogencarbonate solution, shake, release the pressure from the build up of CO2 to neutralise remaining acid
add saturated sodium chloride to see organic layer
allow the layers to separate then discard organic layer using separating funnel
run the organic layer into a clean, dry conical flask and add drying agent (anhydrous sodium sulphate) to dry the organic liquid)
carefully decant the liquid into the distillation flask
distil to collect pure product
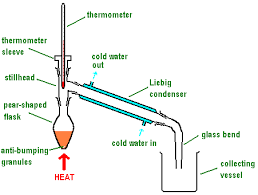
distillation
pour alcohol into round bottomed flask
set up quick fit apparatus
heat with electric heater
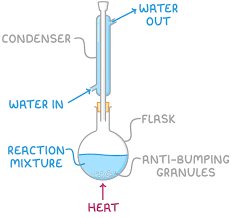
reflux
anti bumping granuals can be added to prevent uneven boiling my making sure small bubbles form
reaction mixture placed in round bottomed flask
flask is connected to condenser which is cooled by a constant water stream
reaction mixture is heater and vapours condense