12-23 Endocrine System
1/53
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
54 Terms
General Principles of the Humoral Control of the Physiological Functions
Hormones are chemical messengers produced by glands in the endocrine system and are released into the bloodstream to target organs and tissues, influencing various physiological functions.
Telecrine (endocrine) hormones are released into the blood and act at a distance (e.g. cortisol, insulin)
Paracrine factors diffuse through interstitial fluid to nearby, different cells (e.g. nitric oxide, prostaglandins)
Autocrine signals act back on the same cell; cytokines can work in any of these modes
Specificity: Hormones exert their effects on specific target cells that possess the appropriate receptors. This ensures that hormonal signals are directed and effective.
Feedback Mechanisms: Many hormonal systems operate on feedback loops, primarily negative feedback, which helps maintain balance. For example, increased levels of a hormone may inhibit further secretion from the gland that produces it.
Concentration and Timing: The effects of hormones depend on their concentration in the bloodstream and the timing of their release. Pulsatile release can enhance or diminish the response of target cells.
Telecrinia and Paracrinia
Telecrinia (Endocrine Signaling)
Hormones secreted into the bloodstream and acting on distant target cells.
Example: Cortisol – produced by the adrenal cortex; circulates in the blood to affect liver (gluconeogenesis), immune cells (anti-inflammatory), adipose tissue (lipolysis), etc
Paracrinia (Paracrine Signaling)
Chemical messengers diffuse locally through interstitial fluid to affect nearby cells.
Examples
Nitric oxide (NO) – released by endothelial cells; diffuses to nearby vascular smooth muscle cells causing vasodilation.
Prostaglandin E₂ (PGE₂) – released by endometrial cells; stimulates nearby uterine muscle contraction.
Classification, Synthesis and Mechanism of Action of the Hormones
Peptide Hormones: These are composed of amino acids and include insulin and glucagon. They are synthesized in ribosomes and processed in the endoplasmic reticulum and stored in vesicles before being secreted.
Steroid Hormones: Derived from cholesterol, these hormones (e.g., cortisol, testosterone) are synthesized in the adrenal cortex and gonads. They are released immediately after synthesizing because they can easily pass through cell membranes due to their lipophilic nature.
Amine Hormones: These are derived from amino acids and can have properties similar to either peptide or steroid hormones:
Catecholamines e.g. epinephrine and norepinephrine are synthesized from the amino acid tyrosine
Thyroid hormones e.g. thyroxine [T4] and triiodothyronine [T3] are synthesized from the amino acid tyrosine in the thyroid gland.
Serotonin is synthesized from the amino acid tryptophan in the enterochromaffin cells of the gastrointestinal tract and in serotonergic neurons in the brain.
Melatonin is synthesized from serotonin in the pineal gland
Control on the Hormone Secretion
Feedback Control: Negative feedback loops are crucial for maintaining hormone levels within a specific range. For example, high levels of thyroid hormones inhibit further release from the thyroid gland.
Hormonal Interactions: Hormones can influence each other's activity through synergistic or antagonistic effects.
For instance, insulin and glucagon have opposing actions on blood glucose levels.
Growth Hormone (GH) and Insulin-like Growth Factor (IGF-1), which work together for growth and development
Estrogen and Progesterone, which regulate the menstrual cycle and pregnancy
Glucagon and glucocorticoids work together to increase blood glucose levels during fasting
Environmental Factors: External stimuli such as stress, light exposure, and nutritional status can influence hormone secretion patterns. For example, stress can increase cortisol levels, affecting metabolism.
Hypothalamic-Neurohypophysial System
Hypothalamus: Located at the base of the brain, the hypothalamus serves as a control center for many autonomic functions. It synthesizes hormones that are transported to the posterior pituitary.
Posterior Pituitary Gland: Unlike the anterior pituitary, which produces its own hormones, the posterior pituitary stores and releases hormones produced in the hypothalamus. The connection between these two structures is primarily neural, hence the term "neurohypophysial."
Oxytocin
Vasopressin (Antidiuretic Hormone, ADH)
Neurosecretion
Neurosecretion refers to the process by which nerve cells (neurons) secrete hormones into the bloodstream.
Hormone Synthesis: Hormones are synthesized in neurosecretory cells located in specific nuclei of the hypothalamus, particularly the supraoptic and paraventricular nuclei.
Transport: These hormones are transported down axons to the posterior pituitary gland, where they are stored in nerve terminals until needed.
Release: When stimulated by appropriate signals (e.g., nerve impulses), these hormones are released into the bloodstream.
Oxytocin
Function: Oxytocin is involved in several physiological functions, including stimulating uterine contractions during childbirth and promoting milk ejection during breastfeeding.
Role in Behavior: It also plays a role in social bonding and emotional responses.
Regulation:
Released in response to cervical stretching during labor or nipple stimulation during breastfeeding.
Positive feedback mechanism: The more oxytocin released, the stronger uterine contractions become, further stimulating its release.
Physiological Effects:
Stimulates uterine contractions during labor, facilitating childbirth.
Promotes milk ejection from mammary glands during lactation.
Enhances emotional bonding between individuals, particularly between mothers and infants.
Vasopressin
Function: Vasopressin regulates water balance in the body by promoting water reabsorption in the kidneys, thus concentrating urine and reducing urine volume.
Role in Blood Pressure Regulation: It also has vasoconstrictive properties that can help increase blood pressure during times of low blood volume or dehydration.
Regulation:
Triggered by increased plasma osmolality (concentration of solutes) or decreased blood volume/pressure.
Osmoreceptors in the hypothalamus detect changes in osmolality and stimulate ADH release when necessary.
Baroreceptors located in blood vessels sense changes in blood pressure and can also influence ADH secretion.
Physiological Effects:
Increases water reabsorption in kidney tubules, leading to reduced urine output and increased blood volume.
Constricts blood vessels, which can raise blood pressure during stress or dehydration.
Hypothalamic-Adenohypophysial System
Hypothalamus: Located at the base of the brain, the hypothalamus serves as a master regulator of endocrine function. It produces several releasing and inhibiting hormones that control the secretion of hormones from the anterior pituitary.
Anterior Pituitary Gland: This gland produces its own hormones in response to signals from the hypothalamus. It is connected to the hypothalamus via a specialized blood vessel system known as the hypothalamic-pituitary portal system, which allows for efficient transport of hormones.
Growth Hormone (GH)
Thyroid-Stimulating Hormone (TSH)
Adrenocorticotropic Hormone (ACTH)
Luteinizing Hormone (LH)
Follicle-Stimulating Hormone (FSH)
Prolactin (PRL)
Growth Hormone (GH)
Function: Stimulates growth and cell reproduction. It promotes protein synthesis and fat breakdown while inhibiting glucose uptake in some tissues.
Regulation: Secretion is stimulated by Growth Hormone-Releasing Hormone (GHRH) from the hypothalamus and inhibited by Somatostatin (growth hormone-inhibiting hormone) based on feedback from blood glucose levels and other factors.
Physiological Effects: Stimulates growth of bones and muscles. Increases protein synthesis and mobilizes fats for energy.
Thyroid-Stimulating Hormone (TSH)
Function: Stimulates the thyroid gland to produce thyroid hormones (T3 and T4), which regulate metabolism, energy levels, and growth.
Regulation: Released in response to Thyrotropin-Releasing Hormone (TRH) from the hypothalamus. TRH stimulates TSH release; high levels of thyroid hormones provide negative feedback to reduce TRH secretion.
Physiological Effects:
Increase BMR (basal metabolic rate) by stimulating the metabolism of carbohydrates, fats, and proteins
Stimulate the growth of bones and tissues
By increasing metabolic activity, thyroid hormones contribute to thermogenesis (heat production) in the body
T3 increases heart rate and enhances cardiac muscle contractility, leading to increased cardiac output.
T3 and T4 play a role in lipid metabolism by promoting the breakdown of cholesterol and increasing the clearance of low-density lipoprotein (LDL) from the bloodstream
They stimulate glycogenolysis (the breakdown of glycogen to glucose) in the liver, increasing blood glucose levels when needed
They promote gluconeogenesis (the production of glucose from non-carbohydrate sources).
Adequate levels of thyroid hormones are important for regular menstrual cycles in women. Hypothyroidism can lead to irregularities in menstrual function.
Adrenocorticotropic Hormone (ACTH)
Function: Stimulates the adrenal cortex to produce cortisol, a hormone involved in stress response, metabolism, and immune function.
Regulation: Triggered by Corticotropin-Releasing Hormone (CRH) from the hypothalamus. CRH stimulates ACTH release; cortisol levels provide negative feedback to inhibit further ACTH secretion.
Physiological Effects: Stimulates cortisol release from the adrenal glands, which helps manage stress responses and affects metabolism.
Luteinizing Hormone (LH) and Follicle-Stimulating Hormone (FSH)
Function: Both are gonadotropins that regulate reproductive processes. LH stimulates ovulation and testosterone production, while FSH promotes follicle development in ovaries and sperm production in testes.
Regulation: Controlled by Gonadotropin-Releasing Hormone (GnRH) from the hypothalamus. GnRH stimulates their release; sex steroid hormones (estrogen/testosterone) provide feedback inhibition on GnRH secretion.
Physiological Effects: Regulate menstrual cycles in females and spermatogenesis in males. Influence secondary sexual characteristics.
Prolactin (PRL)
Function: Promotes milk production in lactating women and has roles in reproductive health.
Regulation: Its secretion is primarily inhibited by Dopamine from the hypothalamus. Stress or suckling can stimulate its release despite this inhibition.
Physiological Effects: Essential for milk production after childbirth. Plays a role in reproductive health and behaviour.
Functional Morphology of the Thyroid Gland
The thyroid gland is a butterfly-shaped endocrine gland located in the anterior neck, just below the larynx and in front of the trachea.
It consists of two lobes connected by a narrow isthmus.
Follicles: The thyroid is composed of numerous spherical structures called follicles, which are filled with colloid, a protein-rich substance containing thyroglobulin. Follicles are the functional units responsible for hormone production.
Follicular Cells: These epithelial cells line the follicles and are responsible for synthesizing thyroid hormones (thyroxine [T4] and triiodothyronine [T3]).
Parafollicular Cells (C Cells): Located between the follicles, these cells produce calcitonin, a hormone involved in calcium homeostasis.
The thyroid gland requires iodine to synthesize its hormones, making iodine an essential nutrient for proper thyroid function.
Iodine-Containing Thyroid Hormones
Thyroxine (T4):
Composed of four iodine atoms.
It is the primary hormone secreted by the thyroid gland and is converted into T3 in peripheral tissues.
Triiodothyronine (T3):
Contains three iodine atoms.
Although produced in smaller quantities than T4, T3 is more biologically active and has a more potent effect on metabolism.
Synthesis of Thyroid Hormones
Iodide Uptake: Follicular cells actively transport iodide from the bloodstream into the thyroid gland.
Thyroglobulin Synthesis: Thyroglobulin is synthesized in the follicular cells and secreted into the colloid.
Iodination: Iodide is oxidized and attached to tyrosine residues on thyroglobulin, forming monoiodotyrosine (MIT) and diiodotyrosine (DIT).
Coupling: MIT and DIT combine to form T3 and T4 within the colloid.
Release: When stimulated by thyroid-stimulating hormone (TSH), thyroglobulin is taken back into follicular cells, where it is broken down to release T3 and T4 into circulation.
Physiological Effects of the Thyroid Hormones
Metabolism: They increase basal metabolic rate (BMR), enhancing oxygen consumption and heat production.
Growth and Development: Essential for normal growth, development, and maturation of tissues, particularly in children.
Cardiovascular Effects: Increase heart rate and cardiac output, enhancing blood flow to tissues.
Nervous System: Influence cognitive functions, mood, and overall brain health.
Regulation of Secretion of the Thyroid Hormones
Thyroid-Stimulating Hormone (TSH): Released from the anterior pituitary gland in response to Thyrotropin-Releasing Hormone (TRH) from the hypothalamus. Increased levels of TSH stimulate the synthesis and release of T3 and T4.
Negative Feedback Mechanism: Elevated levels of T3 and T4 inhibit TRH and TSH secretion, maintaining hormone levels within a normal range.
Hyperthyroidism and Hypothyroidism
Hyperthyroidism
An overproduction of thyroid hormones leads to an accelerated metabolism.
Causes:
Graves' disease (an autoimmune disorder).
Toxic nodular goiter or thyroiditis.
Symptoms: Weight loss, increased heart rate, anxiety, heat intolerance, sweating, tremors, and goiter (enlarged thyroid).
Hypothyroidism
Insufficient production of thyroid hormones results in a slowed metabolism.
Causes:
Hashimoto's thyroiditis (an autoimmune disorder).
Iodine deficiency or surgical removal of the thyroid.
Symptoms: Weight gain, fatigue, cold intolerance, depression, dry skin, hair loss, and goiter.
Other Disorders
Goiter: Enlargement of the thyroid gland due to insufficient iodine intake or other factors affecting hormone production.
Thyroid Nodules: Abnormal growths within the thyroid that can be benign or malignant; some may produce excess hormones leading to hyperthyroidism.
Functional Morphology of Adrenal Glands
The adrenal glands are small, triangular-shaped glands located on top of each kidney.
They are composed of two distinct regions, each responsible for producing different types of hormones
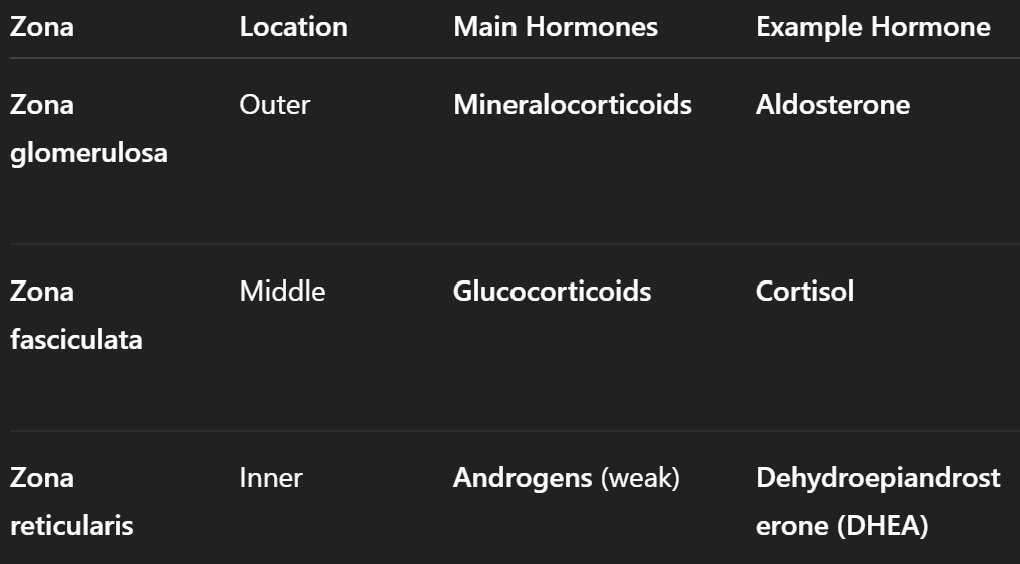
Adrenal Cortex: The outer layer of the adrenal gland, which is further divided into three zones:
Zona Glomerulosa: Produces mineralocorticoids (e.g., aldosterone).
Zona Fasciculata: Produces glucocorticoids (e.g., cortisol).
Zona Reticularis: Produces androgens (e.g., dehydroepiandrosterone [DHEA]).
Adrenal Medulla: The inner part of the adrenal gland that functions as an endocrine organ and is primarily responsible for producing catecholamines (epinephrine and norepinephrine).
Hormones of the Adrenal Medulla
Epinephrine (Adrenaline):
A major hormone involved in the body's "fight or flight" response.
It is released in larger quantities than norepinephrine.
Norepinephrine (Noradrenaline):
Functions similarly to epinephrine but has a slightly different role in the stress response.
Physiological Effects and Control of Secretion of Adrenaline/Epinephrine and Noradrenaline/Norepinephrine
Increased Heart Rate: Both hormones stimulate the heart, increasing cardiac output.
Bronchodilation: They relax bronchial muscles, improving airflow to the lungs.
Increased Blood Glucose Levels: They promote glycogenolysis (the breakdown of glycogen to glucose) in the liver, providing quick energy.
Increased Blood Flow to Muscles: Blood vessels in skeletal muscles dilate, enhancing blood flow during physical activity.
Decreased Blood Flow to Non-Essential Organs: Blood vessels in the digestive system constrict, redirecting blood flow to areas that need it more during stress.
Control of Secretion:
Sympathetic Nervous System Activation: Stressful stimuli activate the sympathetic nervous system, leading to stimulation of the adrenal medulla.
Hypothalamic Control: The hypothalamus releases corticotropin-releasing hormone (CRH), which indirectly influences adrenal medulla activity through its effects on the adrenal cortex.
Hormones of Adrenal Cortex
Physiological Effects and Control of Secretion of Glucocorticoids - Cortisol
Metabolic Effects:
Increases blood glucose levels by promoting gluconeogenesis (the formation of glucose from non-carbohydrate sources) in the liver.
Enhances protein catabolism and fat metabolism, providing energy during stress.
Anti-inflammatory Effects:
Inhibits immune responses and reduces inflammation by suppressing the activity of immune cells.
Stress Response:
Prepares the body to respond to stressors by mobilizing energy resources and modulating immune function.
Control of Secretion:
Adrenocorticotropic Hormone (ACTH): Released from the anterior pituitary gland in response to corticotropin-releasing hormone (CRH) from the hypothalamus. ACTH stimulates cortisol production in the adrenal cortex.
Negative Feedback Mechanism: Elevated cortisol levels inhibit both CRH and ACTH secretion, maintaining hormone levels within a normal range.
Pharmacological Effects of Glucocorticoids
Glucocorticoids are commonly used in medicine for their anti-inflammatory and immunosuppressive properties.
Treatment of Inflammatory Conditions: Glucocorticoids are used to manage conditions such as asthma, arthritis, and allergic reactions due to their ability to reduce inflammation.
Immunosuppression: They are used in organ transplantation to prevent rejection by suppressing immune responses.
Hormonal Replacement Therapy: In cases of adrenal insufficiency (e.g., Addison's disease), glucocorticoids are administered to replace deficient hormones.
Mineralocorticoids - Aldosterone
Aldosterone plays a crucial role in regulating electrolyte balance and blood pressure
Sodium Retention: Aldosterone promotes sodium reabsorption in the kidneys, leading to increased blood volume and blood pressure.
Potassium Excretion: It enhances potassium excretion into urine, helping maintain proper potassium levels in the body.
Control of Secretion of Aldosterone
Renin-Angiotensin-Aldosterone System (RAAS): Low blood pressure or low sodium levels stimulate renin release from the kidneys, leading to a cascade that ultimately increases aldosterone secretion.
Renin = An enzyme secreted by the juxtaglomerular cells of the kidneys
Renin converts angiotensinogen (produced by the liver) into angiotensin I.
Angiotensin I = An inactive precursor that is converted to angiotensin II by the action of angiotensin-converting enzyme (ACE), primarily in the lungs.
Angiotensin II = A potent vasoconstrictor that increases blood pressure by constricting blood vessels. Stimulates the release of aldosterone from the adrenal cortex.
Aldosterone = A mineralocorticoid hormone produced by the adrenal glands
Plasma Potassium Levels: Elevated potassium levels directly stimulate aldosterone release.
Adrenal Androgens
Adrenal sex hormones are produced mainly in the zona reticularis of the adrenal cortex and include androgens such as dehydroepiandrosterone (DHEA) and androstenedione.
Development of Secondary Sexual Characteristics:
Androgens contribute to the development of male secondary sexual characteristics, such as increased muscle mass and body hair growth.
In females, they play a role in libido and can influence hair growth patterns.
Precursor for Sex Steroids:
Adrenal androgens serve as precursors for sex hormones such as testosterone and estrogen, which are produced primarily in the testes and ovaries, respectively.
Influence on Metabolism:
Adrenal sex hormones can affect fat distribution and muscle mass, contributing to overall metabolic health.
Control of Secretion:
Adrenocorticotropic Hormone (ACTH):
ACTH stimulates the zona reticularis to produce adrenal androgens. This regulation is less precise than that for glucocorticoids or mineralocorticoids but still plays a significant role.
Feedback Mechanisms:
Levels of circulating sex hormones can influence ACTH secretion through negative feedback loops, although this feedback mechanism is more pronounced for gonadal hormones than for adrenal androgens.
Abnormalities of Adrenocortical Secretion
Cushing's Syndrome
caused by excessive cortisol production, which can result from adrenal tumors, pituitary adenomas (Cushing's disease), or ectopic ACTH production.
Pathological Effects:
Obesity: Central obesity with a characteristic "moon face" and "buffalo hump."
Skin Changes: Thinning skin, easy bruising, and purple striae (stretch marks).
Metabolic Effects: Hyperglycemia (high blood sugar), insulin resistance, and increased risk of diabetes.
Muscle Weakness: Proximal muscle weakness due to protein catabolism.
Psychological Effects: Mood swings, depression, and cognitive difficulties.
Addison's Disease
insufficient production of adrenal hormones, particularly cortisol and aldosterone, usually due to autoimmune destruction of the adrenal cortex.
Pathological Effects:
Fatigue and Weakness: Chronic fatigue and muscle weakness due to low cortisol levels.
Weight Loss and Anorexia: Unintentional weight loss and decreased appetite.
Hypotension: Low blood pressure due to decreased aldosterone leading to sodium loss.
Hyperpigmentation: Darkening of the skin, particularly in areas exposed to sunlight, due to increased ACTH levels stimulating melanocyte activity.
Electrolyte Imbalance: Hyponatremia (low sodium) and hyperkalemia (high potassium) due to aldosterone deficiency.
Hyperaldosteronism (Conn's Syndrome)
excessive secretion of aldosterone, often due to an adrenal adenoma or hyperplasia.
Pathological Effects:
Hypertension: Elevated blood pressure due to increased sodium reabsorption and water retention.
Hypokalemia: Low potassium levels leading to muscle weakness, cramps, and arrhythmias.
Metabolic Alkalosis: Increased bicarbonate levels due to hydrogen ion loss.
Adrenal Insufficiency Can be primary (Addison's disease) or secondary (due to pituitary failure). Symptoms include fatigue, weakness, weight loss, low blood pressure, and electrolyte imbalances.
Endocrine Functions of the Pancreas - Type of Hormones
The endocrine pancreas is a critical component of the body's metabolic regulation, primarily through the secretion of hormones that control blood glucose levels.
The pancreas has both exocrine (digestive enzymes) and endocrine functions.
The endocrine part consists of clusters of cells known as islets of Langerhans, which contain several types of hormone-producing cells.
Insulin
Glucagon
Somatostatin
Pancreatic Polypeptide
Physiological Effects and Regulation of Secretion of Insulin
Source: Produced by beta cells in the islets of Langerhans.
Physiological Effects:
Lowers blood glucose levels by promoting glucose uptake in cells, especially muscle and adipose tissue.
Stimulates glycogenesis (conversion of glucose to glycogen) in the liver.
Enhances fat storage by promoting lipogenesis and inhibiting lipolysis.
Regulation of Secretion:
Secretion is stimulated by elevated blood glucose levels, certain amino acids, and gastrointestinal hormones (e.g., GLP-1).
Inhibited by low blood glucose levels.
Physiological Effects and Regulation of Secretion of Glucagon
Source: Produced by alpha cells in the islets of Langerhans.
Physiological Effects:
Increases blood glucose levels by promoting glycogenolysis (breakdown of glycogen to glucose) and gluconeogenesis (production of glucose from non-carbohydrate sources) in the liver.
Regulation of Secretion:
Secreted when blood glucose levels are low.
Stimulated by certain amino acids
Inhibited by insulin
Physiological Effects and Regulation of Secretion of Somatostatin
Source: Produced by delta cells in the islets of Langerhans.
Physiological Effects: Inhibits the release of both insulin and glucagon, helping to regulate the overall balance between these hormones.
Regulation of Secretion: Released in response to elevated blood glucose and amino acid levels.
Physiological Effects and Regulation of Secretion of Pancreatic Polypeptide
Source: Produced by PP cells in the islets of Langerhans.
Physiological Effects: Regulates pancreatic secretions and gastrointestinal motility.
Regulation of Secretion: Stimulated by protein-rich meals and fasting.
Diseases of the Endocrine Pancreas
Diabetes Mellitus:
Type 1 Diabetes: An autoimmune disorder resulting in the destruction of beta cells, leading to little or no insulin production. Patients require insulin therapy.
Type 2 Diabetes: Characterized by insulin resistance and relative insulin deficiency. Management includes lifestyle changes, oral medications, and sometimes insulin.
Hypoglycemia: Abnormally low blood glucose levels, which can occur due to excessive insulin production or administration, leading to symptoms like sweating, confusion, and fainting.
Insulinomas: Rare tumors of the pancreas that secrete excess insulin, causing recurrent hypoglycemia.
Calcium-Phosphorus Homeostasis
Parathyroid hormone (PTH): raises [Ca²⁺] by bone resorption, renal Ca²⁺ re‑absorption & phosphate loss, and activating renal 1‑α‑hydroxylase to produce 1,25‑(OH)₂‑D₃ .
1,25‑dihydroxycholecalciferol (vitamin D₃): increases intestinal Ca²⁺/PO₄³⁻ absorption and, with PTH, mobilises bone mineral .
Calcitonin (thyroid C‑cells): minor acute antagonist to PTH, inhibits osteoclastic resorption when plasma Ca²⁺ is high
Physiological Effects and Regulation of Secretion of Parathyroid Hormone (PTH)
Source: Secreted by the parathyroid glands.
Physiological Effects:
Increases blood calcium levels by stimulating osteoclast activity (bone resorption), increasing renal tubular reabsorption of calcium, and promoting conversion of vitamin D to its active form (calcitriol).
Decreases phosphate reabsorption in the kidneys, leading to increased phosphate excretion.
Regulation of Secretion: Secreted in response to low blood calcium levels; secretion decreases when calcium levels rise.
Physiological Effects and Regulation of Secretion of Calcitonin
Source: Produced by parafollicular cells (C cells) in the thyroid gland.
Physiological Effects:
Lowers blood calcium levels by inhibiting osteoclast activity and promoting calcium deposition in bones.
Increases renal excretion of calcium and phosphate.
Regulation of Secretion: Released in response to high blood calcium levels; its role is less critical compared to PTH.
Physiological Effects and Regulation of Secretion of Calcitriol (Active Vitamin D)
Source: Formed from vitamin D3 (cholecalciferol) through a series of conversions in the liver and kidneys.
Physiological Effects:
Increases intestinal absorption of calcium and phosphate from food.
Works synergistically with PTH to mobilize calcium from bones.
Regulation of Secretion: Stimulated by PTH and low serum calcium levels; inhibited when calcium levels are adequate.
Impairment of Calcium-Phosphorus Homeostasis
Hyperparathyroidism: Excessive secretion of PTH leads to elevated blood calcium levels (hypercalcemia), which can cause bone loss, kidney stones, and neurological symptoms.
Hypoparathyroidism: Insufficient PTH secretion results in low blood calcium levels (hypocalcemia), causing muscle cramps, spasms (tetany), and potentially life-threatening cardiac issues.
Vitamin D Deficiency/Rickets/Osteomalacia: Insufficient vitamin D leads to impaired calcium absorption, resulting in weak bones in children (rickets) or adults (osteomalacia).
Paget's Disease: A disorder characterized by abnormal bone remodeling due to excessive osteoclast activity often associated with increased PTH activity.
Osteoporosis: A condition where decreased bone density occurs due to an imbalance between bone resorption and formation, often influenced by hormonal changes including PTH.
Male Reproductive System - Physiology and Spermatogenesis
primarily responsible for the production of sperm and the secretion of male hormones (androgens).
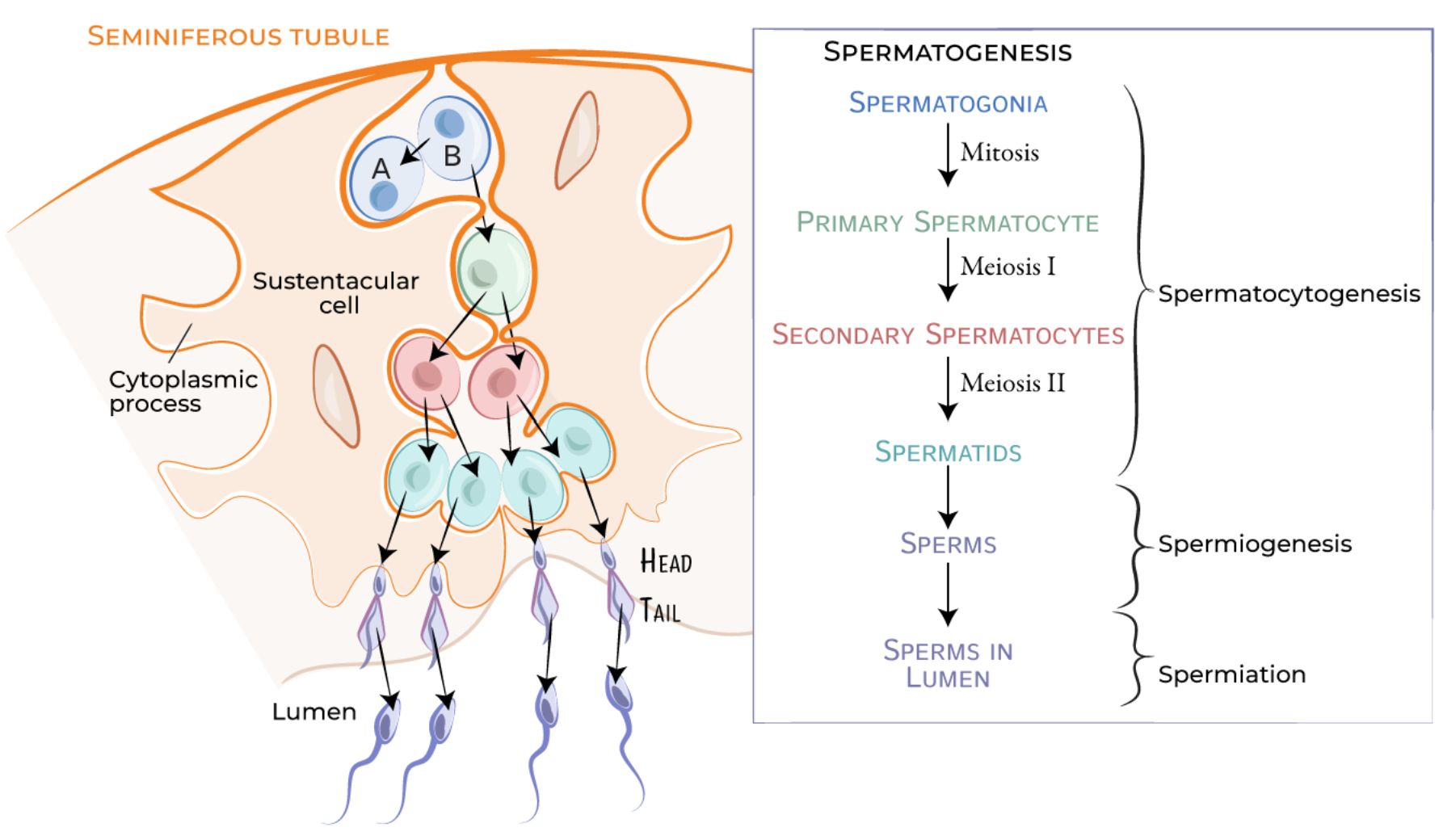
Spermatogenesis is the process by which sperm cells are produced in the testes.
Spermatogonial Phase:
Begins with spermatogonia (germ cells) undergoing mitosis to produce primary spermatocytes.
Meiotic Phase:
Primary spermatocytes undergo meiosis to form secondary spermatocytes, which further divide to produce spermatids.
Spermiogenesis:
Spermatids undergo morphological changes to become mature spermatozoa. This includes the development of a flagellum and condensation of nuclear material.
The entire process takes approximately 74 days and occurs within the seminiferous tubules of the testes.
Male sex hormones (androgens) – types, physiologic effects and control of secretion
Types of Androgens:
Testosterone is the primary androgen, but other androgens include dihydrotestosterone (DHT) and androstenedione.
Physiological Functions:
Development of male secondary sexual characteristics (e.g., facial hair, deep voice).
Regulation of libido (sexual drive).
Promotion of spermatogenesis.
Maintenance of muscle mass and bone density.
Regulation of Secretion:
Testosterone secretion is regulated by luteinizing hormone (LH) from the anterior pituitary, which stimulates Leydig cells in the testes.
Follicle-stimulating hormone (FSH), which stimulates Sertoli cells in the testes, also plays a role by supporting spermatogenesis in conjunction with testosterone.
Erection
Triggered by sexual arousal, leading to increased blood flow into the penis due to vasodilation.
The release of nitric oxide (NO) in response to stimulation causes smooth muscle relaxation in penile arteries.
Ejaculation
Involves two phases:
emission (movement of sperm into the urethra)
expulsion (forceful release through rhythmic contractions)
This process is coordinated by both sympathetic and parasympathetic nervous systems.
Number of sperm cells in an ejaculate > 60 × 106 /ml
Female Reproductive System - Physiology and Oogenesis
responsible for producing ova (eggs)
facilitating fertilization
supporting foetal development during pregnancy
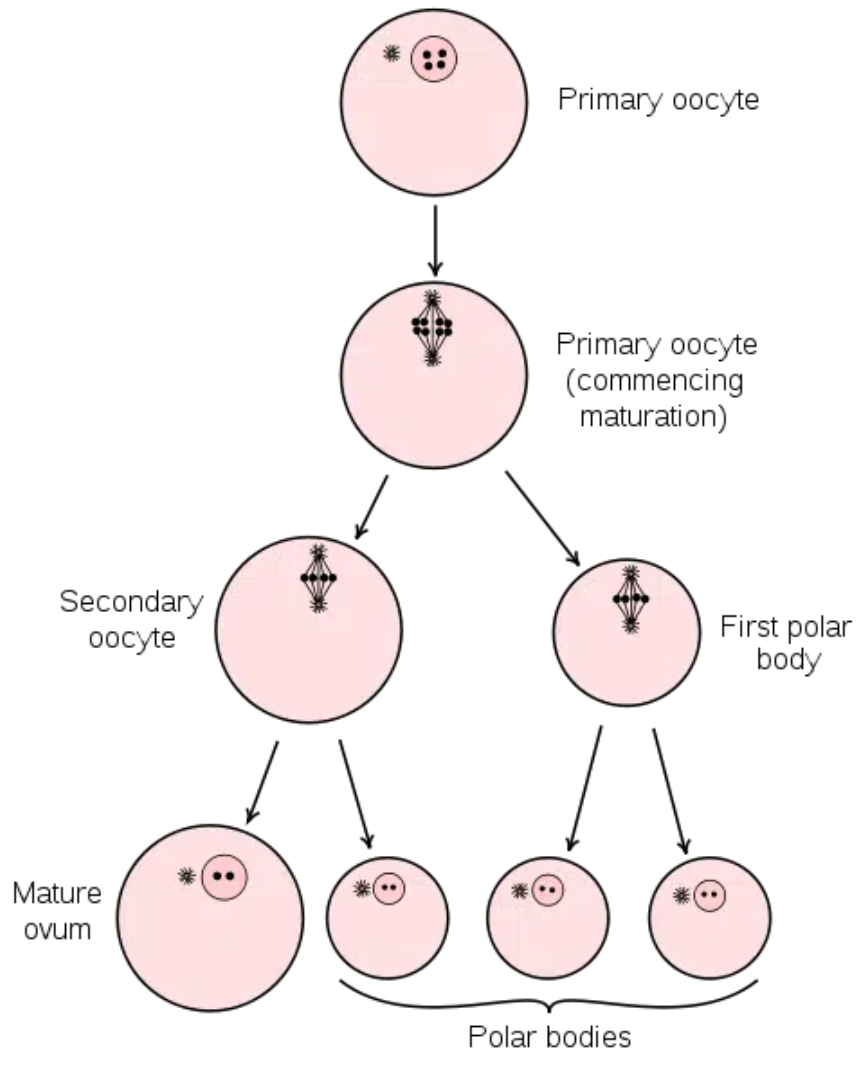
Oogenesis is the process by which oocytes (egg cells) are produced in the ovaries:
Oocyte Development:
Begins before birth with oogonia developing into primary oocytes, which enter meiosis but pause until puberty.
At puberty, primary oocytes resume meiosis during each menstrual cycle, leading to secondary oocyte formation.
Ovulation:
The release of a mature secondary oocyte from the ovary occurs approximately every 28 days during the menstrual cycle.
Female sex hormones (estradiol and progesterone) – types, physiologic effects and control of secretion.
Estrogens:
Types: Estradiol is the most potent form.
Physiological Functions:
Promote development of female secondary sexual characteristics.
Regulate menstrual cycle and prepare the endometrium for potential implantation.
Regulation of Secretion:
Stimulated by follicle-stimulating hormone (FSH) from the anterior pituitary.
Progesterone:
Physiological Functions:
Prepares the endometrium for implantation after ovulation.
Maintains pregnancy by inhibiting uterine contractions.
Regulation of Secretion:
Secreted by the corpus luteum after ovulation; its levels drop if pregnancy does not occur, leading to menstruation.
Regulation of the Female Monthly Rhythm
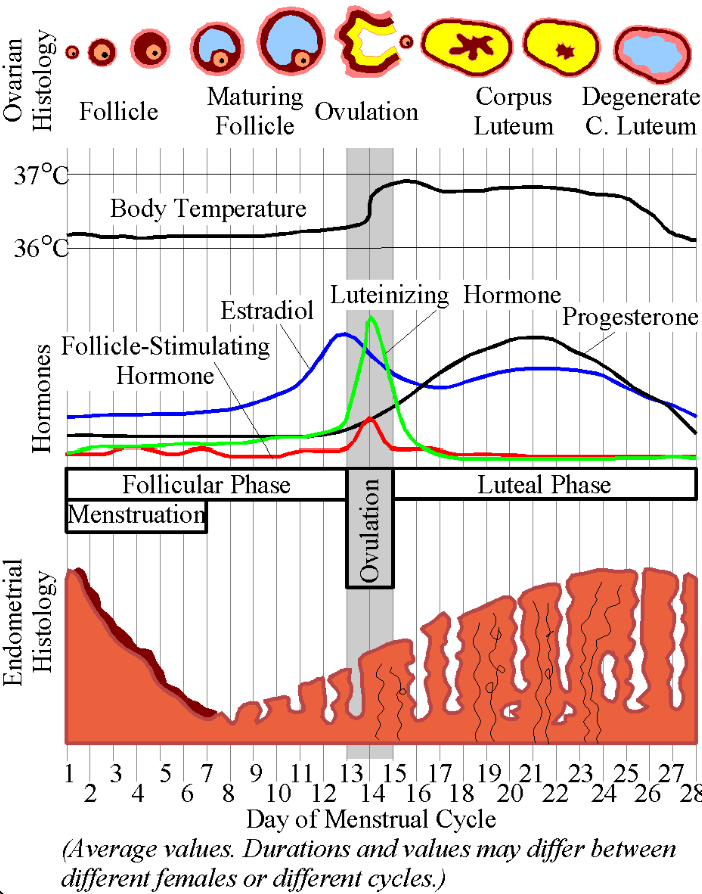
The menstrual cycle typically lasts about 28 days (21-31) and consists of several phases:
Follicular Phase (days 0‑14)
FSH stimulates follicle growth; estrogen levels rise as follicles mature.
Pulsatile GnRH drives modest FSH and LH.
FSH stimulates a cohort of follicles
Rising estradiol from the dominant follicle negatively feeds back on FSH but primes the pituitary
Ovulatory Phase:
A surge in LH triggers ovulation, releasing a secondary oocyte.
Sustained high estradiol flips to positive feedback, triggering a sharp LH (and lesser FSH) surge that induces ovulation 36 h later
Luteal Phase (days 14‑28)
The corpus luteum forms from the ruptured follicle, secreting progesterone and estrogen.
If fertilization does not occur, hormone levels drop, leading to menstruation.
Corpus luteum secretes progesterone ± estradiol; these suppress GnRH/FSH/LH.
Without hCG, luteolysis lowers progesterone → endometrial shedding and GnRH pulses rise, starting a new cycle.
Menstrual bleeding 3-8 days (usually 4-5 days)
Pregnancy and Lactation
Pregnancy:
Begins with fertilization when a sperm cell successfully penetrates an ovum. The resulting zygote implants in the uterine wall.
Birth:
Triggered by hormonal changes that initiate labor contractions; oxytocin plays a key role in stimulating uterine contractions during labor.
Lactation:
Prolactin stimulates milk production in response to suckling, while oxytocin facilitates milk ejection from mammary glands.
Tests for Early Pregnancy
Urine Tests: Detect human chorionic gonadotropin (hCG), a hormone produced shortly after implantation.
Blood Tests: Measure hCG levels more accurately; can detect pregnancy earlier than urine tests.
The Galli-Mainini test is a historical immunological test used for the early detection of pregnancy by identifying the presence of human chorionic gonadotropin (hCG) in a woman's urine or serum.
The Galli-Mainini test is designed to detect hCG, a hormone produced by the placenta shortly after implantation of a fertilized egg in the uterus.
A urine or blood sample is collected from the woman suspected of being pregnant.
The test involves mixing the sample with specific reagents that react with hCG.
Historically, this test utilized animal models (such as rabbits) where the urine was injected into the animal, and then the animal's physiological response was observed. If pregnancy was present, it would cause changes in the animal's ovaries (e.g., ovarian enlargement).
A positive result indicates the presence of hCG and suggests that the woman is pregnant.
A negative result indicates that hCG is not detectable, suggesting that the woman is not pregnant.
Immunological tests are critical tools in diagnosing various diseases by detecting specific antibodies or antigens in biological samples.
Urine Pregnancy Tests (Home Tests)
When urine is applied to the test strip, it interacts with these antibodies.
The woman collects a urine sample, typically using the first-morning urine for a higher concentration of hCG.
The sample is applied to a test strip or device.
If hCG is present, it binds to the antibodies on the strip, leading to a visible change (such as a color change or line appearing).
A positive result indicates pregnancy, while a negative result suggests that hCG is not detectable.
Blood Pregnancy Tests:
Qualitative Blood Test: Similar to urine tests, this test determines whether hCG is present in the blood but does not measure its quantity.
Quantitative Blood Test (Beta hCG Test): This test measures the exact level of hCG in the blood and can provide more information about the pregnancy's status (e.g., confirming early pregnancy or monitoring for potential complications).
Epiphysis (Pineal Gland) with Endocrine Functions
The epiphysis, commonly known as the pineal gland, is a small endocrine gland located in the brain.
It plays a crucial role in regulating circadian rhythms and reproductive hormones.
Hormone Produced:
Melatonin: The primary hormone secreted by the pineal gland, melatonin is synthesized from serotonin during the night.
Physiological Effects:
Regulates sleep-wake cycles by promoting sleepiness in response to darkness.
Influences seasonal reproductive functions in some animals by modulating gonadal activity.
Thymus with Endocrine Functions
The thymus is an organ located in the upper chest, playing a vital role in the immune system, particularly during childhood.
Hormones Produced:
Thymosin, Thymopoietin, and other thymic hormones.
Physiological Effects:
Stimulate the development and differentiation of T lymphocytes (T cells), which are crucial for adaptive immunity.
Promote immune responses and help establish self-tolerance to prevent autoimmune diseases.
Non-Endocrine Organs with Endocrine Functions
Several non-endocrine organs also produce hormones or hormone-like substances that exert regulatory effects on various physiological processes:
Adipose Tissue:
Produces hormones such as leptin (regulates energy balance and appetite) and adiponectin (enhances insulin sensitivity).
Muscle Tissue:
Produces myokines like irisin, which promotes energy expenditure and glucose metabolism.
Gastrointestinal Tract:
Produces various hormones (e.g., gastrin, secretin, cholecystokinin) that regulate digestion, appetite, and metabolism.
Tissue Hormones - Types, Physiological Effects and Control of Secretion
A tissue hormone is a locally acting chemical messenger that is:
Produced on demand by cells in a tissue,
Acts locally (paracrine or autocrine action),
Not transported via blood in significant amounts (unlike endocrine hormones),
Often rapidly degraded, limiting their action to nearby cells.
Not stored in glands; synthesized as needed.
Act within the same tissue where they are produced.
Derived from membrane lipids (e.g. arachidonic acid) or amino acids
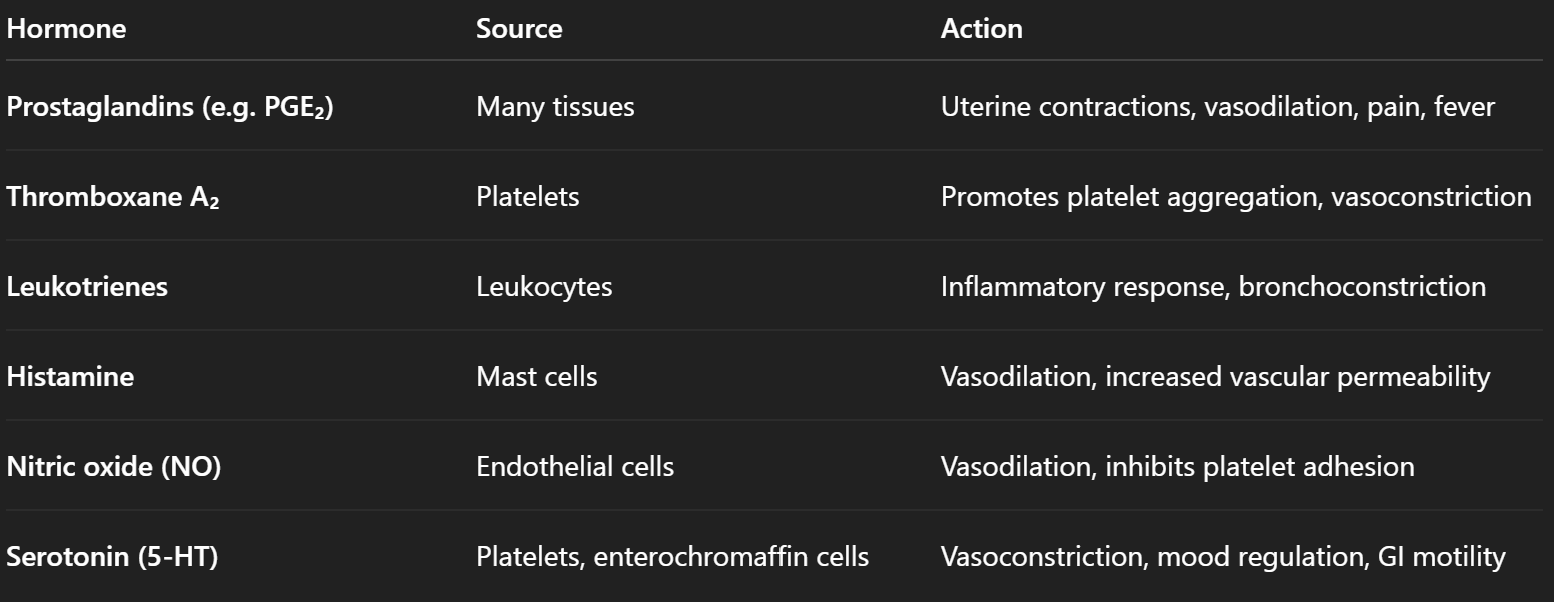
Prototypical eicosanoids (prostaglandins, thromboxanes, leukotrienes)
Prostaglandins (PGs) modulate smooth‑muscle tone (e.g. uterine contractions), vasodilation/constriction, platelet aggregation, fever and pain; seminal PGs enhance cervical mucus penetrability and reverse uterine peristalsis to aid sperm transport
Control: rate‑limiting cyclo‑oxygenase activity rises with mechanical trauma, cytokines or other hormones (e.g. oxytocin in labour). Rapid local degradation limits their range to paracrine/autocrine distances.
Cytokines:
Small proteins released by cells that affect the behavior of other cells.
Effects: Involved in immune responses, inflammation, and cell signaling. Examples include interleukins and tumor necrosis factor (TNF).
Growth Factors:
Proteins that stimulate cell growth, proliferation, and differentiation.
Effects: Play critical roles in wound healing and tissue repair. Examples include epidermal growth factor (EGF) and vascular endothelial growth factor (VEGF).
Myokines:
Hormones produced by muscle cells during contraction.
Effects: Influence metabolism and insulin sensitivity; examples include irisin.
Adipokines:
Hormones secreted by adipose tissue.
Effects: Regulate energy metabolism and inflammation; examples include leptin and adiponectin.